Sunday driver interacts with two distinct classes of axonal organelles - PubMed (original) (raw)
Sunday driver interacts with two distinct classes of axonal organelles
Namiko Abe et al. J Biol Chem. 2009.
Abstract
The extreme polarized morphology of neurons poses a challenging problem for intracellular trafficking pathways. The distant synaptic terminals must communicate via axonal transport with the cell soma for neuronal survival, function, and repair. Multiple classes of organelles transported along axons may establish and maintain the polarized morphology of neurons, as well as control signaling and neuronal responses to extracellular cues such as neurotrophic or stress factors. We reported previously that the motor-binding protein Sunday Driver (syd), also known as JIP3 or JSAP1, links vesicular axonal transport to injury signaling. To better understand syd function in axonal transport and in the response of neurons to injury, we developed a purification strategy based on anti-syd antibodies conjugated to magnetic beads to identify syd-associated axonal vesicles. Electron microscopy analyses revealed two classes of syd-associated vesicles of distinct morphology. To identify the molecular anatomy of syd vesicles, we determined their protein composition by mass spectrometry. Gene Ontology analyses of each vesicle protein content revealed their unique identity and indicated that one class of syd vesicles belongs to the endocytic pathway, whereas another may belong to an anterogradely transported vesicle pool. To validate these findings, we examined the transport and localization of components of syd vesicles within axons of mouse sciatic nerve. Together, our results lead us to propose that endocytic syd vesicles function in part to carry injury signals back to the cell body, whereas anterograde syd vesicles may play a role in axonal outgrowth and guidance.
Figures
FIGURE 1.
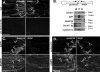
syd and the molecular motors are associated with vesicles of distinct size. A, schematic diagram of the synaptosome preparation method, modified from Ref. . B, mouse brain cortex was fractionated by differential centrifugation to obtain a synaptosome-enriched fraction LP2. syd, JNK, and the molecular motors are present in the LP2 fraction, which is enriched with the synaptic vesicle markers synaptophysin and VAMP. The lack of enrichment of these proteins in the LP2 fraction is consistent with these proteins being associated with various organelles and the presence of large soluble populations. C, following sedimentation of the LP2 fraction on a sucrose velocity gradient, two distinct vesicles pools were obtained. Small vesicles containing synaptic vesicle markers were detected in fractions 3 and 4 (SVP). Larger vesicles containing the presynaptic membrane protein SNAP25 were detected in fractions 8, 9, and 10 (LVP). syd, JNK, and the molecular motor proteins were detected in both small and large vesicles fractions. D, electron microscopic analysis of pooled fractions 3 and 4 revealed the presence of synaptic vesicles of 30–50 nm in diameter together with other organelles of up to 100 nm in diameter. E, EM analysis of pooled fractions 8, 9, and 10 revealed a more heterogeneous profile of organelles, vesicles, and tubules ranging from 100 to 500 nm. In D and E, bar = 400 nm; in the inset, bar = 200 nm.
FIGURE 2.
Electron microscopic analysis of immunoisolated syd vesicles. SVP (A–C) or LVP (D–F) were incubated with magnetic beads coated with the indicated antibody (Ab). A, control beads coated with preimmune serum were devoid of any membrane profiles. B, vesicles from fractions 3 and 4 adsorbed to the anti-synaptotagmin beads revealed the expected profile for 30–50-nm synaptic vesicles. C, membranes from fractions 3 and 4 adsorbed to anti-syd beads were composed of 100–200 nm small single membrane vesicle (arrowheads) and tubules (arrows). D, control beads coated with preimmune serum and incubated with the LVP were devoid of any membrane profiles. E, membranes adsorbed to anti-syd beads from the LVP were composed primarily of large, multivesicular vesicles of up to 500 nm. These membrane profiles are similar to endosomes adsorbed on anti-Rab5 beads. In A–F, bar = 200 nm.
FIGURE 3.
Western blot analysis of immunoisolated syd vesicles. Vesicles immunoisolated from SVP (A and C) or LVP (B and D) using the indicated antibodies were analyzed by Western blot (WB). Efficiency of isolation was estimated at ∼20% for both SVP and LVP fractions. A, small syd vesicles were distinct from synaptic vesicles. Immunoisolation with syd antibodies did not contain components of synaptic vesicles such as synaptotagmin, synaptophysin, or VAMP, and reversely, immunoisolated synaptic vesicles on anti-synaptotagmin-coated beads did not contain syd. IP, immunoprecipitation; pre-i, preimmune serum used as a negative control; IN, percentage of input material for the immunoisolation; GFP, green fluorescent protein. B, large syd vesicles contained the endosomal markers syntaxin 13, VAMP3, and clathrin, but no detectable VAMP or Na+/K+ exchanger. The motor proteins KIF5C and anti-p150_Glued_ were detected in large syd vesicles, but no significant amount of the other kinesin motor KIF3A was detected, in agreement with syd being an adaptor for the conventional kinesin-1 motor. C and D, reverse immunoisolations were performed using antibodies against proteins identified by mass spectrometry. C, beads coated with anti-SNAP29 isolated vesicles containing syd. D, immunoisolation using the indicated endosomal markers isolated syd, indicating that syd in part resides on endosomes. The input material is 10% for all immunoisolations, except for VAMP, which is 20%.
FIGURE 4.
Mass spectrometry and Gene Ontology analysis. A, the protein composition of synaptic and syd-associated vesicles was determined by mass spectrometry analysis. Spectra counting, i.e. the number of peaks detected for a particular protein, was used as a relative quantification between each sample and their negative control. The synaptic vesicle protein composition provided a positive control to determine the minimal -fold difference in spectra counts between syd immunoisolations and their respective negative controls. Thus, only proteins with a minimum of a 2-fold higher value in spectra count relative to their respective control were included in the final list (
supplemental Data S1
). A Venn diagram was created to indicate the protein composition overlap between the three types of vesicles. B, proteins within each vesicle category were connected to biological process annotations provided by the Gene Ontology (GO) Consortium. Based on the hierarchical structure of the Gene Ontology annotations, the probability that each immediate daughter term (a p value) be linked to the number of selected genes by chance was calculated. This analysis revealed that small and large syd vesicles are distinct from each other and that both are distinct from synaptic vesicles. Out of 142 GO terms in the biological process category with at least one of the three categories having a p value inferior to 0.05, we selected 25 representative GO terms to build the illustrated table. The complete tables are available as
supplemental Data S2, S3, and S4
for the biological process, cellular component, and molecular function categories, respectively. Red, p value ≤0.001; orange, p value >0.001 and ≤0.01; light orange, p value >0.01 and ≤0.05; white, p value >0.05. C, the 39 and 194 proteins unique to small and large syd vesicles, respectively, were grouped in functional categories. Fewer RNA processing- and signaling-related proteins and more trafficking and endocytosis and synaptic-related proteins were found in large syd vesicles.
FIGURE 5.
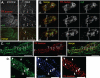
Axonal transport of syd vesicle components. Sciatic nerves were ligated unilaterally at the midpoint and processed for immunofluorescence microscopy or SDS-PAGE and Western blot analysis. A, syd accumulates on both the proximal and the distal side of the ligation site, as expected. Ab, antibody. B, ligated and contralateral unligated sciatic nerves were dissected. and extracts were analyzed by Western blot with the indicated antibodies. SNAP29 and synaptotagmin VII (SytVII), two proteins identified on the small syd vesicles, accumulated mostly on the proximal side, indicative of anterograde transport. Low levels are also detected to some extent on the distal sides, similarly to amyloid precursor protein (APP), a well established anterograde marker. The synaptotagmin antibody recognizes several isoforms, as indicated. Syntaxin 13, identified on the large syd vesicles, was detected on both the proximal and the distal sides, indicating that these proteins are transported in both anterograde and retrograde directions. Tubulin is used as a loading control. Ul, unligated; P, proximal; D, distal. C and D, SNAP29 and synaptotagmin VII are found mostly on the proximal side (C), and syntaxin 13 and VAMP3 are found on both sides (D), similarly to syd. In A, C, and D, bar = 100 μm.
FIGURE 6.
syd localization with in vivo labeled endosomes. The endocytic pathway within sensory neurons was labeled by subcutaneous injection of the tracer Texas Red dextran in the mouse rear leg footpad. A sciatic nerve ligation concomitant with dye injection was performed to increase the number of labeled structures accumulating distal to the ligation site. The sciatic nerve was dissected 24 h after injection, fixed, and embedded in cryomold. Longitudinal sections were analyzed by immunofluorescence. A, low magnification images showed syd accumulation in Texas Red dextran (TR dextran)-positive axons (arrowheads). B and C, Nikon Optigrid structured illumination microscopy (B) or confocal microscopy (C) followed by deconvolution showed that Texas Red dextran puncta partially co-localize with syd. Three consecutive sections in the z plane are shown in B. D, triple labeling immunostaining showed that syd-dextran-positive structures also contained the endosomal protein VAMP3/cellubrevin, further supporting the notion that syd resides at least in part on axonal endosomes. In A, bar = 100 μm; in B–D, bar = 5 μm.
Comment in
- Beware of neuronal Sunday drivers: Sunday Driver interacts with two distinct classes of axonal organelles.
[No authors listed] [No authors listed] J Biol Chem. 2009 Dec 11;284(50):e99968. J Biol Chem. 2009. PMID: 20050128 Free PMC article. No abstract available.
Similar articles
- Sunday Driver links axonal transport to damage signaling.
Cavalli V, Kujala P, Klumperman J, Goldstein LS. Cavalli V, et al. J Cell Biol. 2005 Feb 28;168(5):775-87. doi: 10.1083/jcb.200410136. J Cell Biol. 2005. PMID: 15738268 Free PMC article. - Beware of neuronal Sunday drivers: Sunday Driver interacts with two distinct classes of axonal organelles.
[No authors listed] [No authors listed] J Biol Chem. 2009 Dec 11;284(50):e99968. J Biol Chem. 2009. PMID: 20050128 Free PMC article. No abstract available. - Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility.
Sun F, Zhu C, Dixit R, Cavalli V. Sun F, et al. EMBO J. 2011 Jul 12;30(16):3416-29. doi: 10.1038/emboj.2011.229. EMBO J. 2011. PMID: 21750526 Free PMC article. - Keeping Neuronal Cargoes on the Right Track: New Insights into Regulators of Axonal Transport.
Miller KG. Miller KG. Neuroscientist. 2017 Jun;23(3):232-250. doi: 10.1177/1073858416648307. Epub 2016 May 6. Neuroscientist. 2017. PMID: 27154488 Review. - What is the importance of multivesicular bodies in retrograde axonal transport in vivo?
Weible MW 2nd, Hendry IA. Weible MW 2nd, et al. J Neurobiol. 2004 Feb 5;58(2):230-43. doi: 10.1002/neu.10318. J Neurobiol. 2004. PMID: 14704955 Review.
Cited by
- Is amyloid binding alcohol dehydrogenase a drug target for treating Alzheimer's disease?
Borger E, Aitken L, Du H, Zhang W, Gunn-Moore FJ, Yan SS. Borger E, et al. Curr Alzheimer Res. 2013 Jan;10(1):21-9. Curr Alzheimer Res. 2013. PMID: 22742981 Free PMC article. Review. - Syd/JIP3 and JNK signaling are required for myonuclear positioning and muscle function.
Schulman VK, Folker ES, Rosen JN, Baylies MK. Schulman VK, et al. PLoS Genet. 2014 Dec 18;10(12):e1004880. doi: 10.1371/journal.pgen.1004880. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25522254 Free PMC article. - JIP3 interacts with dynein and kinesin-1 to regulate bidirectional organelle transport.
Celestino R, Gama JB, Castro-Rodrigues AF, Barbosa DJ, Rocha H, d'Amico EA, Musacchio A, Carvalho AX, Morais-Cabral JH, Gassmann R. Celestino R, et al. J Cell Biol. 2022 Aug 1;221(8):e202110057. doi: 10.1083/jcb.202110057. Epub 2022 Jul 13. J Cell Biol. 2022. PMID: 35829703 Free PMC article. - Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury.
van Niekerk EA, Tuszynski MH, Lu P, Dulin JN. van Niekerk EA, et al. Mol Cell Proteomics. 2016 Feb;15(2):394-408. doi: 10.1074/mcp.R115.053751. Epub 2015 Dec 22. Mol Cell Proteomics. 2016. PMID: 26695766 Free PMC article. Review. - Opposing roles for GSK3β and ERK1-dependent phosphorylation of huntingtin during neuronal dysfunction and cell death in Huntington's disease.
Krzystek TJ, Rathnayake R, Zeng J, Huang J, Iacobucci G, Yu MC, Gunawardena S. Krzystek TJ, et al. Cell Death Dis. 2025 Apr 22;16(1):328. doi: 10.1038/s41419-025-07524-0. Cell Death Dis. 2025. PMID: 40263294 Free PMC article.
References
- Salinas S., Bilsland L. G., Schiavo G. (2008) Curr. Opin. Cell Biol. 20, 445–453 - PubMed
- Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S. A., Rammner B., Gräter F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. (2006) Cell 127, 831–846 - PubMed
- Burger P. M., Mehl E., Cameron P. L., Maycox P. R., Baumert M., Lottspeich F., De Camilli P., Jahn R. (1989) Neuron 3, 715–720 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM035252/GM/NIGMS NIH HHS/United States
- R01 EY007042/EY/NEI NIH HHS/United States
- GM35252/GM/NIGMS NIH HHS/United States
- R01 NS060709/NS/NINDS NIH HHS/United States
- EY07042/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases