The human insulin gene is part of a large open chromatin domain specific for human islets - PubMed (original) (raw)
The human insulin gene is part of a large open chromatin domain specific for human islets
Vesco Mutskov et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Knowledge of how insulin (INS) gene expression is regulated will lead to better understanding of normal and abnormal pancreatic beta cell function. We have mapped histone modifications over the INS region, coupled with an expression profile, in freshly isolated islets from multiple human donors. Unlike many other human genes, in which active modifications tend to be concentrated within 1 kb around the transcription start site, these marks are distributed over the entire coding region of INS as well. Moreover, a region of approximately 80 kb around the INS gene, which contains the {tyrosine hydroxylase (TH)-(INS)-insulin-like growth factor 2 antisense (IGF2AS)-insulin-like growth factor 2 (IGF2)} gene cluster, unusually is marked by almost uniformly elevated levels of histone acetylation and H3K4 dimethylation, extending both downstream into IGF2 and upstream beyond the TH gene. This is accompanied by islet specific coordinate expression with INS of the neighboring TH and IGF2 genes. The presence of islet specific intergenic transcripts suggests their possible function in the maintenance of this unusual large open chromatin domain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
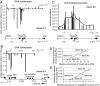
The TH-INS-IGF2AS-IGF2 gene cluster. The TH, INS, IGF2AS and IGF2 genes are found together at the telomeric end of the short arm of chromosome 11 (11p15.5). Large gene-free regions flank this gene cluster; each is ≈100 kb long and the positions of the next few genes on both sites are indicated. TH, INS and IGF2 are transcribed in the same direction. The position of the VNTR polymorphic region between the TH and INS genes and Alu repeated sequence between INS and IGF2 are indicated.
Fig. 2.
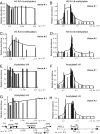
ChIP analysis of methylation of histone H3 K4 over the INS gene locus in islets. (A) ChIP of histone H3K4 dimethylation over the 11-kb region neighboring the INS gene in islets, donor 1 (black) and control HeLa cells (white). The data from Fig. 2_B_ are presented at higher resolution. (B) ChIP of dimethylation of H3K4 over the 250 kb on 11p15.5 including the TH-INS-IGF2AS-IGF2 cluster in islets from donor 1 (black) and hBM-MSC cells (white) as a control. Each bar represents data from an individual primer/TaqMan probe set: islets in black, control hBM-MSC cells in white. In parallel, these DNA samples were amplified with primers specific for the GAPDH gene as an internal control. The relative abundance of each sequence is the ratio of its concentration in the IP fraction to that in the input. This was normalized to data for GAPDH, with the latter set equal to 1. (C) ChIP of histone H3K4 trimethylation over the 11-kb region. The data from Fig. 2_D_ from donor 2 are shown at higher resolution. (D) ChIP of histone H3K4 trimethylation over the 250-kb region in islets. (E) The tetra-acetylated histone H4 data from donor 1 (black) and HeLa cells (white) are shown at higher resolution for the 11-kb region. (F) ChIP of tetra-acetylated histone H4 in islets from donor 1 (black) and control hBM-MSC (white). (G) ChIP of di-acetylation of histone H3 over the 11-kb region near the INS gene in islets from donor 3. (H) Analysis of the levels of histone H3 di-acetylation in islets from donor 3.
Fig. 3.
ChIP of histone modifications over the INS gene locus in islets from a single donor. (A) ChIP of H3K4 dimethylation over the 250-kb region from donor 4. The analyses were performed as described in Fig. 2. To compare the profiles of distribution of each individual histone modification in a single donor, the highest point of relative abundance was set to 1 for all four histone modifications. (B) ChIP of tetra-acetylated histone H4 over the 250-kb region. (C) ChIP of di-acetylated histone H3. (D) ChIP of histone H3K27 trimethylation.
Fig. 4.
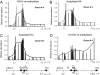
Expression analyses in islets and control cells. (A) QRT-PCR on RNA isolated from islets from six donors, labeled with letters A–F corresponding to donors 1, 5, 10, 8, 3, and 11 respectively. HeLa cells (G) and human fibroblasts (H) were controls. Each bar represents the abundance of mRNA relative to GAPDH mRNA. (B) Correlation among levels of expression of INS, TH, IGF2-AS, IGF2, and β actin in islets. The levels of expression of the genes in the six preparations were plotted against the corresponding levels of expression of the PDX1.
Fig. 5.
DNA methylation and intergenic transcription over the INS gene locus in islets. (A) Sonicated genomic DNA from islets (donor 3) or HeLa cells was immunoprecipitated with an antibody against 5-methylcytidine. Data were analyzed as described for
Fig. S5_A_
. The differences between the relative abundance for each sequence in islets and in HeLa cells are plotted. (B) DNA methylation over the 11-kb region. Data from Fig. 5_A_ are presented at higher resolution. (C) QRT-PCR of islet RNAs from donor 6. The same primers/TaqMan probes sets were used as for ChIP experiments; only those spanning the intergenic regions are shown. Bars represent relative abundance of intergenic transcript, relative to GAPDH mRNA. (D) Correlation between levels of INS gene expression and of intergenic transcription at position 2140955 on Chr:11p15.5. Data from five different donors were compared. The x axis shows the levels of INS gene expression estimated as described in Fig. 4. The y axis shows the levels of intergenic transcription at position 2140955 estimated as described above. (E) Correlation between levels of intergenic transcription at position 2140955 and the extent of histone H3K4 dimethylation at that position. The x axis shows the extent of histone modification estimated as described in Fig. 2. The y axis data are the same as in D.
Similar articles
- Autoimmunity against INS-IGF2 protein expressed in human pancreatic islets.
Kanatsuna N, Taneera J, Vaziri-Sani F, Wierup N, Larsson HE, Delli A, Skärstrand H, Balhuizen A, Bennet H, Steiner DF, Törn C, Fex M, Lernmark Å. Kanatsuna N, et al. J Biol Chem. 2013 Oct 4;288(40):29013-23. doi: 10.1074/jbc.M113.478222. Epub 2013 Aug 9. J Biol Chem. 2013. PMID: 23935095 Free PMC article. - Diabetes susceptibility at IDDM2 cannot be positively mapped to the VNTR locus of the insulin gene.
Doria A, Lee J, Warram JH, Krolewski AS. Doria A, et al. Diabetologia. 1996 May;39(5):594-9. doi: 10.1007/BF00403307. Diabetologia. 1996. PMID: 8739920 - Chromatin structure, epigenetic mechanisms and long-range interactions in the human insulin locus.
Xu Z, Lefevre GM, Felsenfeld G. Xu Z, et al. Diabetes Obes Metab. 2012 Oct;14 Suppl 3(Suppl 3):1-11. doi: 10.1111/j.1463-1326.2012.01645.x. Diabetes Obes Metab. 2012. PMID: 22928559 Free PMC article. Review. - Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation.
Omori K, Mitsuhashi M, Todorov I, Rawson J, Shiang KD, Kandeel F, Mullen Y. Omori K, et al. Transplantation. 2010 Jan 27;89(2):146-54. doi: 10.1097/TP.0b013e3181c4218d. Transplantation. 2010. PMID: 20098276 Free PMC article. - Emerging roles of GLIS3 in neonatal diabetes, type 1 and type 2 diabetes.
Wen X, Yang Y. Wen X, et al. J Mol Endocrinol. 2017 Feb;58(2):R73-R85. doi: 10.1530/JME-16-0232. Epub 2016 Nov 29. J Mol Endocrinol. 2017. PMID: 27899417 Review.
Cited by
- Early-life physical activity reverses metabolic and Foxo1 epigenetic misregulation induced by gestational sleep disturbance.
Mutskov V, Khalyfa A, Wang Y, Carreras A, Nobrega MA, Gozal D. Mutskov V, et al. Am J Physiol Regul Integr Comp Physiol. 2015 Mar 1;308(5):R419-30. doi: 10.1152/ajpregu.00426.2014. Epub 2015 Jan 7. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25568076 Free PMC article. - Mapping of INS promoter interactions reveals its role in long-range regulation of SYT8 transcription.
Xu Z, Wei G, Chepelev I, Zhao K, Felsenfeld G. Xu Z, et al. Nat Struct Mol Biol. 2011 Mar;18(3):372-8. doi: 10.1038/nsmb.1993. Epub 2011 Feb 20. Nat Struct Mol Biol. 2011. PMID: 21336277 Free PMC article. - Mysterious long noncoding RNAs and their relationships to human disease.
Li W, Wang YY, Xiao L, Ding J, Wang L, Wang F, Sun T. Li W, et al. Front Mol Biosci. 2022 Nov 2;9:950408. doi: 10.3389/fmolb.2022.950408. eCollection 2022. Front Mol Biosci. 2022. PMID: 36406273 Free PMC article. Review. - Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes.
Sandovici I, Hammerle CM, Ozanne SE, Constância M. Sandovici I, et al. Cell Mol Life Sci. 2013 May;70(9):1575-95. doi: 10.1007/s00018-013-1297-1. Epub 2013 Mar 6. Cell Mol Life Sci. 2013. PMID: 23463236 Free PMC article. Review. - Insulin-secreting β cells require a post-genomic concept.
Jiang FX, Morahan G. Jiang FX, et al. World J Diabetes. 2016 May 25;7(10):198-208. doi: 10.4239/wjd.v7.i10.198. World J Diabetes. 2016. PMID: 27226815 Free PMC article. Review.
References
- Rodriguez S, Gaunt TR, Day IN. Molecular genetics of human growth hormone, insulin-like growth factors and their pathways in common disease. Hum Genet. 2007;122:1–21. - PubMed
- Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. - PubMed
- Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic β-cells. Biochem J. 2008;415:1–10. - PubMed
- Sproul D, Gilbert N, Bickmore WA. The role of chromatin structure in regulating the expression of clustered genes. Nat Rev Genet. 2005;6:775–781. - PubMed
- Caron H, et al. The human transcriptome map: Clustering of highly expressed genes in chromosomal domains. Science. 2001;291:1289–1292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous