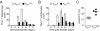Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity - PubMed (original) (raw)
. 2009 Oct 13;106(41):17469-74.
doi: 10.1073/pnas.0907448106. Epub 2009 Sep 24.
Zachary A Borman, Lydie Cassard, Luca Gattinoni, Rosanne Spolski, Zhiya Yu, Luis Sanchez-Perez, Pawel Muranski, Steven J Kern, Carol Logun, Douglas C Palmer, Yun Ji, Robert N Reger, Warren J Leonard, Robert L Danner, Steven A Rosenberg, Nicholas P Restifo
Affiliations
- PMID: 19805141
- PMCID: PMC2762661
- DOI: 10.1073/pnas.0907448106
Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity
Christian S Hinrichs et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Effector cells derived from central memory CD8(+) T cells were reported to engraft and survive better than those derived from effector memory populations, suggesting that they are superior for use in adoptive immunotherapy studies. However, previous studies did not evaluate the relative efficacy of effector cells derived from naïve T cells. We sought to investigate the efficacy of tumor-specific effector cells derived from naïve or central memory T-cell subsets using transgenic or retrovirally transduced T cells engineered to express a tumor-specific T-cell receptor. We found that naïve, rather than central memory T cells, gave rise to an effector population that mediated superior antitumor immunity upon adoptive transfer. Effector cells developed from naïve T cells lost the expression of CD62L more rapidly than those derived from central memory T cells, but did not acquire the expression of KLRG-1, a marker for terminal differentiation and replicative senescence. Consistent with this KLRG-1(-) phenotype, naïve-derived cells were capable of a greater proliferative burst and had enhanced cytokine production after adoptive transfer. These results indicate that insertion of genes that confer antitumor specificity into naïve rather than central memory CD8(+) T cells may allow superior efficacy upon adoptive transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
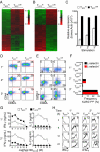
Effector CD8+ T cells derived from naïve or central memory cells acquire cytolytic phenotype and function. (A) Production of IFN-γ by freshly isolated cells in an overnight coculture assay. The error bars represent the standard error of the mean. (B) The number of cell divisions 2 days after peptide stimulation, as determined by CFSE dilution from a FACS histogram. (C) Schematic delineating generation of primary and secondary effector cells from naïve and central memory CD8+ T cells. (D) Cytolytic function of TEFFN and TEFFCM as determined by 51Cr release assay. The target peptide, gp10025–33 or NP366–374 is indicated in parenthesis. (E) Flow cytometric analysis indicating expression of granzyme B and perforin and (F) L-selectin and CD44. The open histograms indicate isotype antibody controls. All figures shown are representative of at least two independent experiments.
Fig. 2.
Naïve and central memory cells confer distinct genetic signatures and developmental programs to their effector cell progeny. Microarray analysis of the gene expression of (A) rested and (B) stimulated TEFFN and TEFFCM. Probe sets with FDR ≤2% and ≥2-fold differences in expression are shown. Red and green colors indicate increased and decreased expression respectively. The scales are log10. (C) Relative expression of Eomes by TEFFN or TEFFCM as determined by real time RT-PCR. The x axis indicates the number of times the cells were stimulated with antigen and IL-2. Error bars represent the standard error of the mean. (D) Flow cytometric determination of the expression of CD62L and CD44 by TEFFN and TEFFCM through serial stimulations. The numbers on the dot plots indicate the frequency of cells with central memory (CD62L+ CD44high) phenotype. (E) Expression of CD62L and KLRG1. Quadrant frequencies are shown. (F) Graph derived from panel E. Frequencies of cells from the CD62L+ or CD62L− fraction of TEFFN or TEFFCM with KLRG1high phenotype. (G) IFN-γ and IL-2 production, in coculture assays, by effector cells generated by primary or secondary stimulation of naïve or central memory cells. (H) Intracellular IFN-γ expression following peptide stimulation of TEFFN or TEFFCM. The mean fluorescence intensities are indicated. All figures shown are representative of at least two independent experiments.
Fig. 3.
Use of effector cells derived from naïve rather than central memory cells improves adoptive cell transfer therapy. (A) Tumor responses following treatment with 4 × 106 effector cells generated from naïve or central memory subsets. All groups except “No treatment” received adjuvant vaccine and IL-2. TEFFN versus TEFFCM, P < 0.01. (_B_) Production of IFN-γ and IL-2 by adoptively transferred cells reisolated from spleens 6 days following infusion. Cell numbers were equalized for the assay. (_C_) Tumor treatment with 7.65 × 105 wild-type or IFN-γ−/− effector cells. TEFFN versus IFN-γ−/− TEFFN, _P_ < 0.05. (_D_) Tumor treatment with 1.05 × 106 wild-type or IL-2−/− effector cells. TEFFN versus IL-2−/− TEFFN, _P_ > 0.05. All figures shown are representative of at least two independent experiments.
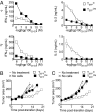
Fig. 4.
Effector cells derived directly from naïve cells possess greater potential for clonal expansion following infusion. (A and B) The number of cells present in spleens and lymph nodes, respectively, following adoptive transfer of TEFFN or TEFFCM. Error bars indicate the standard error of the mean. (n = 4 for each group and each time point). (C) The frequency of transferred cells in recipient spleens displaying KLRG1high phenotype 5 days following infusion. The transverse lines represent the means. P = 0.0024. All figures shown are representative of at least two independent experiments.
Fig. 5.
Genetically engineered open repertoire cells reproduce the central findings of the transgenic mouse model. (A) Elaboration of IFN-γ and IL-2 by TCR transduced naïve- or central memory-derived effector cells. (B) Tumor growth following treatment with transduced effector cells generated by 4 × 106 primary or (C) 2 × 107 secondary stimulation of naïve or central memory cells. All figures shown are representative of at least two independent experiments.
Similar articles
- Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy.
Wu F, Zhang W, Shao H, Bo H, Shen H, Li J, Liu Y, Wang T, Ma W, Huang S. Wu F, et al. Cancer Lett. 2013 Oct 10;339(2):195-207. doi: 10.1016/j.canlet.2013.06.009. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791878 - Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy.
Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Hinrichs CS, et al. Blood. 2011 Jan 20;117(3):808-14. doi: 10.1182/blood-2010-05-286286. Epub 2010 Oct 22. Blood. 2011. PMID: 20971955 Free PMC article. - Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates.
Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Berger C, et al. J Clin Invest. 2008 Jan;118(1):294-305. doi: 10.1172/JCI32103. J Clin Invest. 2008. PMID: 18060041 Free PMC article. - Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy.
Crompton JG, Sukumar M, Restifo NP. Crompton JG, et al. Immunol Rev. 2014 Jan;257(1):264-276. doi: 10.1111/imr.12135. Immunol Rev. 2014. PMID: 24329803 Free PMC article. Review. - Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours?
Perret R, Ronchese F. Perret R, et al. Tissue Antigens. 2008 Sep;72(3):187-94. doi: 10.1111/j.1399-0039.2008.01088.x. Epub 2008 Jul 9. Tissue Antigens. 2008. PMID: 18627571 Review.
Cited by
- Targeting epigenetic regulation and post-translational modification with 5-Aza-2' deoxycytidine and SUMO E1 inhibition augments T-cell receptor therapy.
Kroonen JS, Wouters AK, de Graaf IJ, Remst DFG, Kumar S, Wachsmann TLA, Teunisse AFAS, Roelands JP, de Miranda NFCC, Griffioen M, Heemskerk MHM, Vertegaal ACO. Kroonen JS, et al. J Immunother Cancer. 2024 Sep 26;12(9):e008654. doi: 10.1136/jitc-2023-008654. J Immunother Cancer. 2024. PMID: 39326886 Free PMC article. - Fine-Tuning through Generations: Advances in Structure and Production of CAR-T Therapy.
Zheng Z, Li S, Liu M, Chen C, Zhang L, Zhou D. Zheng Z, et al. Cancers (Basel). 2023 Jul 3;15(13):3476. doi: 10.3390/cancers15133476. Cancers (Basel). 2023. PMID: 37444586 Free PMC article. Review. - Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL.
Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason J, Kipps TJ, Gillenwater HH, Gong L, Yang L, Ogasawara K, Thorpe J, Wierda WG. Siddiqi T, et al. Blood. 2022 Mar 24;139(12):1794-1806. doi: 10.1182/blood.2021011895. Blood. 2022. PMID: 34699592 Free PMC article. Clinical Trial. - Lineage relationship of effector and memory T cells.
Restifo NP, Gattinoni L. Restifo NP, et al. Curr Opin Immunol. 2013 Oct;25(5):556-63. doi: 10.1016/j.coi.2013.09.003. Epub 2013 Oct 20. Curr Opin Immunol. 2013. PMID: 24148236 Free PMC article. Review. - Adoptive T-cell therapy for hematological malignancies using T cells gene-modified to express tumor antigen-specific receptors.
Fujiwara H. Fujiwara H. Int J Hematol. 2014 Feb;99(2):123-31. doi: 10.1007/s12185-013-1493-7. Epub 2013 Dec 19. Int J Hematol. 2014. PMID: 24352938 Review.
References
- Childs R, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials