Central role of TRAF-interacting protein in a new model of brain sexual differentiation - PubMed (original) (raw)
Central role of TRAF-interacting protein in a new model of brain sexual differentiation
Sudha Krishnan et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Sexually dimorphic brain nuclei underlie gender-specific neural functions and susceptibility to disease, but the developmental basis of dimorphisms is poorly understood. In these studies, we focused on the anteroventral periventricular nucleus (AVPV), a nucleus that is larger in females and critical for the female-typical cyclic surge pattern of luteinizing hormone (LH) release. Sex differences in the size and function of the AVPV result from apoptosis that occurs preferentially in the developing male. To identify upstream pathways responsible for sexual differentiation of the AVPV, we used targeted apoptosis microarrays and in vivo and in vitro follow-up studies. We found that the tumor necrosis factor alpha (TNFalpha)-TNF receptor 2 (TNFR2)-NFkappaB cell survival pathway is active in postnatal day 2 (PND2) female AVPV and repressed in male counterparts. Genes encoding key members of this pathway were expressed exclusively in GABAergic neurons. One gene in particular, TNF receptor-associated factor 2 (TRAF2)-inhibiting protein (trip), was higher in males and it inhibited both TNFalpha-dependent NFkappaB activation and bcl-2 gene expression. The male AVPV also had higher levels of bax and bad mRNA, but neither of these genes was regulated by either TNFalpha or TRIP. Finally, the trip gene was not expressed in the sexually dimorphic nucleus of the preoptic area (SDN-POA), a nucleus in which apoptosis is higher in females than males. These findings form the basis of a new model of sexual differentiation of the AVPV that may also apply to the development of other sexually dimorphic nuclei.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
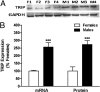
Fig. 1.
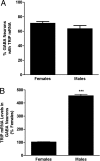
Sex differences in TRIP mRNA and protein levels in AVPV on PND2. QPCR was used to measure TRIP mRNA and Western blot analysis to measure TRIP. (A) Photomicrograph of Western blot results using 4 pools each of female (F1–F4) and male (M1–M4) AVPV with GAPDH as loading control. (B) Graphical depiction of Western and QPCR analyses of TRIP mRNA and protein. Each bar represents mean ± SEM of 4 separate samples, each of which contained AVPV tissue pooled from 4 individual animals that came from at least 3 different litters.***, P < 0.005, significantly different from female.
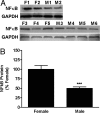
Fig. 2.
Sex differences in nuclear NFκB in AVPV on PND2. Nuclear NFκB-p65 subunit-containing heterodimers were measured in microdissections of AVPV from male and female rat pups collected on PND2. (A) Photomicrographs showing results of Westerns that were measured using densitometry. (B) Graph of results of Western blot analysis. Each bar represents mean ± SEM of 5-6 separate samples, each of which contained AVPV tissue pooled from 4 individual animals that came from at least 3 different litters. Levels were measured using Western blot analysis and GAPDH was used as an internal control. ***, P < 0.0001, significantly different from female.
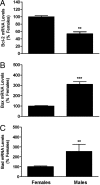
Fig. 3.
Sex differences in mRNAs encoding members of the Bcl2 family. Bcl2 (A), Bad (B), and Bax (C) mRNA levels were measured in microdissections of AVPV from male and female rat pups collected on PND2. Each bar represents mean ± SEM of 4 samples and each sample contained tissue from 4 different animals representing at least 3 different litters. Levels were measured using QPCR. GAPDH was used as an internal control. **, P < 0.01; ***, P < 0.0001, significantly different from female.
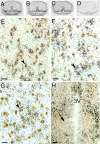
Fig. 4.
Autoradiograms of ISH for TNFR2 (A), TRAF2 (B), and TRIP (C) mRNA hybridized to 35S-labeled cRNA probes in brain POA sections that contain the AVPV of PND2 male rats. (D) Example of signal produced by sense strand control probes (TRIP). Photomicrographs showing results of dual-label ISH studies colocalizing digoxigenin-labeled cRNA probes to GAD mRNA (marker of GABA neurons; brown stain) and 35S-labeled cRNA probes to TNFR2 (E), TRAF2 (F), or TRIP (G) mRNA in POA sections of PND2 males. Arrowheads denote double-labeled cells. (Scale bar, 20 μm.) (H) Note the total absence of signal in the more caudal SDN-POA (denoted by arrow). (Scale bar, 100 μm.)
Fig. 5.
Sex differences in percentage of AVPV GABAergic neurons that contained TRIP mRNA (A) and relative levels of TRIP mRNA in AVPV GABAergic neurons (B). Each bar represents mean ± SEM of values from female (n = 4–5) and male (n = 4–5) rat pups examined on PND2. Data were obtained using computer-assisted image analysis of dual-label ISH studies. We used 35S-labeled cRNA probes for TRIP mRNA and digoxigenin-labeled cRNA probes for GAD mRNA. ***, P < 0.0001, significantly different from female.
Fig. 6.
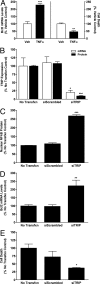
Results of in vitro studies on the effects of TNFα and TRIP in N42 hypothalamic cells. (A) Effects of TNFα or vehicle (Veh) on Bcl2 mRNA levels measured with QPCR and on cell death measured using CytoTox-Fluor cytotoxicity assay. Each bar represents mean ± SEM of 6 samples assayed in duplicate. **, P < 0.005, significantly different from Vehicle controls; ***, P < 0.001. (B) Verification of siRNA knockdown of TRIP mRNA and protein levels. Each bar represents mean ± SEM of 4 samples. (C–E) Effects of TRIP silencing on TNFα-induced NFκB-p65 subunit levels analyzed using Western blots (C), Bcl2 mRNA levels measured with QPCR (D), and cell death (E). In these studies, cells were treated with 100 U of TNFα and comparisons were made among cells without transfection (No Transfxn) or transfection for 24 h with vectors containing AllStars Negative Control siRNA (siScrambled) or vectors containing a combination of 2 siTRIP constructs, S101454649 and S101454635. (C–E) Each bar represents mean ± SEM of at least 4 samples assayed in duplicate. *, P < 0.05, significantly different from No Transfxn and siScrambled controls; **, P < 0.001; ***, P < 0.0001.
Similar articles
- Microarray analysis of neonatal rat anteroventral periventricular transcriptomes identifies the proapoptotic Cugbp2 gene as sex-specific and regulated by estradiol.
Del Pino Sans J, Krishnan S, Aggison LK, Adams HL, Shrikant MM, López-Giráldez F, Petersen SL. Del Pino Sans J, et al. Neuroscience. 2015 Sep 10;303:312-22. doi: 10.1016/j.neuroscience.2015.07.008. Epub 2015 Jul 9. Neuroscience. 2015. PMID: 26166732 - Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction.
Park ES, Choi S, Shin B, Yu J, Yu J, Hwang JM, Yun H, Chung YH, Choi JS, Choi Y, Rho J. Park ES, et al. J Biol Chem. 2015 Apr 10;290(15):9660-73. doi: 10.1074/jbc.M114.609685. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716317 Free PMC article. - Sexual differentiation of the gonadotropin surge release mechanism: a new role for the canonical NfκB signaling pathway.
Petersen SL, Krishnan S, Aggison LK, Intlekofer KA, Moura PJ. Petersen SL, et al. Front Neuroendocrinol. 2012 Jan;33(1):36-44. doi: 10.1016/j.yfrne.2011.06.002. Epub 2011 Jun 28. Front Neuroendocrinol. 2012. PMID: 21741397 Review. - Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats.
Tsukahara S. Tsukahara S. J Neuroendocrinol. 2009 Mar;21(4):370-6. doi: 10.1111/j.1365-2826.2009.01855.x. J Neuroendocrinol. 2009. PMID: 19226350 Review. - Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats.
Tsukahara S, Kakeyama M, Toyofuku Y. Tsukahara S, et al. J Neurobiol. 2006 Nov;66(13):1411-9. doi: 10.1002/neu.20276. J Neurobiol. 2006. PMID: 17013925
Cited by
- Sexual differentiation and development of forebrain reproductive circuits.
Semaan SJ, Kauffman AS. Semaan SJ, et al. Curr Opin Neurobiol. 2010 Aug;20(4):424-31. doi: 10.1016/j.conb.2010.04.004. Epub 2010 May 12. Curr Opin Neurobiol. 2010. PMID: 20471241 Free PMC article. Review. - Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: effects of age and sex.
Ahern TH, Krug S, Carr AV, Murray EK, Fitzpatrick E, Bengston L, McCutcheon J, De Vries GJ, Forger NG. Ahern TH, et al. J Comp Neurol. 2013 Aug 1;521(11):2551-69. doi: 10.1002/cne.23298. J Comp Neurol. 2013. PMID: 23296992 Free PMC article. - Feminization of social play behavior depends on microglia.
VanRyzin JW, Marquardt AE, McCarthy MM. VanRyzin JW, et al. bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608675. doi: 10.1101/2024.08.19.608675. bioRxiv. 2024. PMID: 39229086 Free PMC article. Preprint. - Sex Differences in the Cerebral Collateral Circulation.
Faber JE, Moore SM, Lucitti JL, Aghajanian A, Zhang H. Faber JE, et al. Transl Stroke Res. 2017 Jun;8(3):273-283. doi: 10.1007/s12975-016-0508-0. Epub 2016 Nov 14. Transl Stroke Res. 2017. PMID: 27844273 Free PMC article. - PKCζ phosphorylates TRAF2 to protect against intestinal ischemia-reperfusion-induced injury.
Zhou W, Yao J, Wang G, Chen Z, Li Z, Feng D, Li Y, Qasim W, Tan W, Ning S, Tian X. Zhou W, et al. Cell Death Dis. 2017 Jul 20;8(7):e2935. doi: 10.1038/cddis.2017.310. Cell Death Dis. 2017. PMID: 28726782 Free PMC article.
References
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. - PubMed
- Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Amer J Anat. 1936;58:195–225.
- Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484:279–289. - PubMed
- Ronnekleiv OK, Kelly MJ. Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: Effects of surgically induced persistent estrus. Neuroendocrinology. 1986;43:564–576. - PubMed
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials