Purification and reconstitution of the antigen transport complex TAP: a prerequisite for determination of peptide stoichiometry and ATP hydrolysis - PubMed (original) (raw)
Purification and reconstitution of the antigen transport complex TAP: a prerequisite for determination of peptide stoichiometry and ATP hydrolysis
Meike Herget et al. J Biol Chem. 2009.
Abstract
The transporter associated with antigen processing (TAP) is an essential machine of the adaptive immune system that translocates antigenic peptides from the cytosol into the endoplasmic reticulum lumen for loading of major histocompatibility class I molecules. To examine this ABC transport complex in mechanistic detail, we have established, after extensive screening and optimization, the solubilization, purification, and reconstitution for TAP to preserve its function in each step. This allowed us to determine the substrate-binding stoichiometry of the TAP complex by fluorescence cross-correlation spectroscopy. In addition, the TAP complex shows strict coupling between peptide binding and ATP hydrolysis, revealing no basal ATPase activity in the absence of peptides. These results represent an optimal starting point for detailed mechanistic studies of the transport cycle of TAP by single molecule experiments to analyze single steps of peptide translocation and the stoichiometry between peptide transport and ATP hydrolysis.
Figures
FIGURE 1.
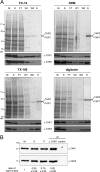
Purification of TAP. A, purification of TAP with different detergents. TAP-containing membranes (5 mg/ml of protein) were solubilized by different detergents and purified by metal affinity chromatography. Aliquots of membranes (M), solubilization (S), flow through (FT), wash with 20 m
m
(W1), wash with 40 m
m
histidine (W2), and elution with 200 m
m
of histidine (E) were analyzed by SDS-PAGE (9%, Coomassie-stained) and immunoblotting using monoclonal α-TAP1 (148.3) and α-TAP2 (435.3) antibodies. B, stoichiometry of TAP. TAP-containing membranes (10 mg/ml protein) were solubilized by 2% digitonin, purified by the His10 tag at the C terminus of TAP1 via metal affinity chromatography, and subsequently immunoprecipitated via monoclonal α-TAP2 (435.3) antibody to isolate exclusively heterodimeric TAP complexes or α-MHC I (HC10) antibody as isotope control. Aliquots of membranes (M), solubilization (S), metal affinity-purified TAP (P), and immunoprecipitated TAP (IP) were analyzed by SDS-PAGE (10%) and immunoblotting using monoclonal α-TAP1 (148.3) and α-TAP2 (435.3) antibodies. The ratio of the TAP1 and TAP2 intensities in each fraction was normalized to the membrane level. The ratios depict the mean of three independent experiments.
FIGURE 2.
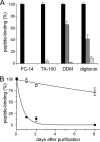
Activity of solubilized TAP. A, stability of TAP during purification. To determine the stability of the TAP complex during purification, crude membranes derived from 1 liter of insect cell culture were solubilized by different detergents and purified by metal affinity chromatography. Subsequently, fractions of crude membranes (black bars), after solubilization (dark gray bars) and purified TAP (light gray bars) containing nominal equal amounts of TAP were incubated with radiolabeled peptide RRYQKSTEL (300 n
m
) in the presence or absence of 200-fold excess of unlabeled peptide. After 15-min incubation at 4 °C, bound peptides were separated by filter assay and quantified by γ-counting. Specific binding to TAP in crude membranes was normalized to 100%. B, long term stability of TAP. To compare the stability and TAP activity over time, TAP purified in DDM (filled circles) or digitonin (open circles) was stored at 4 °C. Peptide binding was analyzed as described in A. Specific peptide binding to TAP at day zero was normalized to 100%. In digitonin, TAP was active with a half-life longer than 16 days, whereas in DDM the half-life was only 10.5 h. Each data point is derived from triplicate measurements. Error bars show the standard deviation.
FIGURE 3.
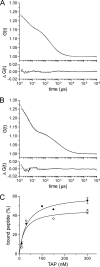
Peptide-binding affinity determined by fluorescence correlation spectroscopy. A, fluorescence auto-correlation of free peptide. Auto-correlation data (gray line) obtained for Alexa-633-peptide RRYCKSTEL (2.5 n
m
) were fitted by a one-component fit (Equation 3, black line) taking a fraction of the triplet state into account (τtriplet = 4.4 μs). A diffusion time (τfree) of 200 μs resulted from the fit. B, fluorescence auto-correlation of TAP-bound peptides. 2.5 n
m
of the Alexa-633-peptide RRYCKSTEL was incubated with 320 n
m
of solubilized and purified TAP for 30 min at 4 °C. The fluorescence correlation spectroscopy measurements were performed at 22 °C. Auto-correlation data (gray line) were fitted by a two-component model assuming fractions of free and bound peptides (Equation 3, black line) taking a fraction of the triplet state into account (τtriplet = 5.7 μs). The diffusion time for free peptide (τfree) was fixed to 200 μs, resulting in a diffusion time of bound peptide (τbound) of 1953 μs. The lower graph displays the residuals of the fit. C, Alexa-488- or Alexa-633-peptides RRYCKSTEL (2.5 n
m
of each) were incubated with increasing concentrations of TAP. Auto-correlation curves were fitted by a two-component model assuming fractions of free and bound peptides. The fraction of bound peptide (%) was plotted against the TAP concentration and fitted by a Langmuir (1:1) isotherm. For the Alexa-488 peptide, and the Alexa-633 peptide a KD of 20 ± 9 n
m
(open circles) and 30 ± 6 n
m
(closed circles) was deduced, respectively.
FIGURE 4.
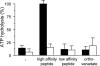
Peptide binding induces ATP hydrolysis of the solubilized TAP complex. 500 n
m
of purified wt (filled bars) or inactive TAP variant (TAP1 K544A/TAP2 K509A, H661A) (open bars) were incubated with 1 m
m
MgATP in the presence or absence of high affinity peptide (2 μ
m
RRYQKSTEL), low affinity peptide (2 μ
m
EPGNTWDED), or _ortho_-vanadate (2 m
m
) in combination with high peptide (2 μ
m
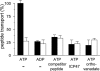
) for 1 h at 32 °C. Release of inorganic phosphate was quantified by the malachite green assay and normalized to the TAP-dependent ATPase activity in the presence of RRYQKSTEL. Data are derived from triplicate measurements. Error bars show the standard deviation.
FIGURE 5.
Functional reconstitution of TAP in proteoliposomes. Proteoliposomes (46 μg of E. coli lipids) containing wt (filled bars) or inactive TAP (open bars; 0.75 μg of each) were incubated in 50 μl with 1 μ
m
RRYC(F)KSTEL in the presence of MgATP or MgADP (3 m
m
of each). The assay was performed in the presence of unlabeled competitor peptide (200 μ
m
RRYQKSTEL), the viral TAP inhibitor ICP47 (30 μ
m
), or _ortho_-vanadate (3 m
m
) as indicated. Data are derived from triplicate measurements. Error bars show the standard deviation.
Similar articles
- Peptides induce ATP hydrolysis at both subunits of the transporter associated with antigen processing.
Chen M, Abele R, Tampé R. Chen M, et al. J Biol Chem. 2003 Aug 8;278(32):29686-92. doi: 10.1074/jbc.M302757200. Epub 2003 May 30. J Biol Chem. 2003. PMID: 12777379 - Analyses of conformational states of the transporter associated with antigen processing (TAP) protein in a native cellular membrane environment.
Geng J, Sivaramakrishnan S, Raghavan M. Geng J, et al. J Biol Chem. 2013 Dec 27;288(52):37039-47. doi: 10.1074/jbc.M113.504696. Epub 2013 Nov 6. J Biol Chem. 2013. PMID: 24196954 Free PMC article. - Kinetic analysis of peptide binding to the TAP transport complex: evidence for structural rearrangements induced by substrate binding.
Neumann L, Tampé R. Neumann L, et al. J Mol Biol. 1999 Dec 17;294(5):1203-13. doi: 10.1006/jmbi.1999.3329. J Mol Biol. 1999. PMID: 10600378 - The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter.
Procko E, O'Mara ML, Bennett WF, Tieleman DP, Gaudet R. Procko E, et al. FASEB J. 2009 May;23(5):1287-302. doi: 10.1096/fj.08-121855. Epub 2009 Jan 27. FASEB J. 2009. PMID: 19174475 Review. - Antigen processing and presentation: TAPping into ABC transporters.
Procko E, Gaudet R. Procko E, et al. Curr Opin Immunol. 2009 Feb;21(1):84-91. doi: 10.1016/j.coi.2009.02.003. Epub 2009 Mar 2. Curr Opin Immunol. 2009. PMID: 19261456 Review.
Cited by
- The lysosomal transporter TAPL has a dual role as peptide translocator and phosphatidylserine floppase.
Park JG, Kim S, Jang E, Choi SH, Han H, Ju S, Kim JW, Min DS, Jin MS. Park JG, et al. Nat Commun. 2022 Oct 4;13(1):5851. doi: 10.1038/s41467-022-33593-2. Nat Commun. 2022. PMID: 36195619 Free PMC article. - Structural basis for heavy metal detoxification by an Atm1-type ABC exporter.
Lee JY, Yang JG, Zhitnitsky D, Lewinson O, Rees DC. Lee JY, et al. Science. 2014 Mar 7;343(6175):1133-6. doi: 10.1126/science.1246489. Science. 2014. PMID: 24604198 Free PMC article. - Epstein-Barr viral BNLF2a protein hijacks the tail-anchored protein insertion machinery to block antigen processing by the transport complex TAP.
Wycisk AI, Lin J, Loch S, Hobohm K, Funke J, Wieneke R, Koch J, Skach WR, Mayerhofer PU, Tampé R. Wycisk AI, et al. J Biol Chem. 2011 Dec 2;286(48):41402-41412. doi: 10.1074/jbc.M111.237784. Epub 2011 Oct 7. J Biol Chem. 2011. PMID: 21984826 Free PMC article. - ATPase activity of human ABCG1 is stimulated by cholesterol and sphingomyelin.
Hirayama H, Kimura Y, Kioka N, Matsuo M, Ueda K. Hirayama H, et al. J Lipid Res. 2013 Feb;54(2):496-502. doi: 10.1194/jlr.M033209. Epub 2012 Nov 19. J Lipid Res. 2013. PMID: 23172659 Free PMC article. - Quantitative real-time analysis of the efflux by the MacAB-TolC tripartite efflux pump clarifies the role of ATP hydrolysis within mechanotransmission mechanism.
Souabni H, Batista Dos Santos W, Cece Q, Catoire LJ, Puvanendran D, Bavro VN, Picard M. Souabni H, et al. Commun Biol. 2021 Apr 22;4(1):493. doi: 10.1038/s42003-021-01997-3. Commun Biol. 2021. PMID: 33888866 Free PMC article.
References
- Schölz C., Tampé R. (2005) J. Bioenerg. Biomembr. 37, 509–515 - PubMed
- Groothuis T. A., Griekspoor A. C., Neijssen J. J., Herberts C. A., Neefjes J. J. (2005) Immunol. Rev. 207, 60–76 - PubMed
- Schmitt L., Tampé R. (2002) Curr. Opin. Struct. Biol. 12, 754–760 - PubMed
- Hollenstein K., Dawson R. J., Locher K. P. (2007) Curr. Opin. Struct. Biol. 17, 412–418 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous