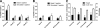Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Dec;32(12):2251-7.
doi: 10.2337/dc09-0773. Epub 2009 Oct 6.
Lisa M Spain, Robert A Wesley, Benigno J Digon 3rd, Alain Baron, Kim Chen, Patric Nelson, H-Michael Dosch, Jerry P Palmer, Barbara Brooks-Worrell, Michael Ring, David M Harlan
Affiliations
- PMID: 19808924
- PMCID: PMC2782986
- DOI: 10.2337/dc09-0773
Randomized Controlled Trial
Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes
Kristina I Rother et al. Diabetes Care. 2009 Dec.
Abstract
Objective: In patients with long-standing type 1 diabetes, we investigated whether improved beta-cell function can be achieved by combining intensive insulin therapy with agents that may 1) promote beta-cell growth and/or limit beta-cell apoptosis and 2) weaken the anti-beta-cell autoimmunity.
Research design and methods: For this study, 20 individuals (mean age 39.5 +/- 11.1 years) with long-standing type 1 diabetes (21.3 +/- 10.7 years) were enrolled in this prospective open-label crossover trial. After achieving optimal blood glucose control, 16 subjects were randomized to exenatide with or without daclizumab. Endogenous insulin production was determined by repeatedly measuring serum C-peptide.
Results: In 85% of individuals with long-standing type 1 diabetes who were screened for participation in this trial, C-peptide levels >or=0.05 ng/ml (0.02 nmol/l) were found. Residual beta-cells responded to physiological (mixed-meal) and pharmacological (arginine) stimuli. During exenatide treatment, patients lost 4.1 +/- 2.9 kg body wt and insulin requirements declined significantly (total daily dose on exenatide 0.48 +/- 0.11 vs. 0.55 +/- 0.13 units x kg(-1) x day(-1) without exenatide; P = 0.0062). No signs of further activation of the underlying autoimmune disease were observed. Exenatide delayed gastric emptying, suppressed endogenous incretin levels, but did not increase C-peptide secretion.
Conclusions: In long-standing type 1 diabetes, which remains an active autoimmune disease even decades after its onset, surviving beta-cells secrete insulin in a physiologically regulated manner. However, the combination of intensified insulin therapy, exenatide, and daclizumab did not induce improved function of these remaining beta-cells.
Trial registration: ClinicalTrials.gov NCT00064714.
Figures
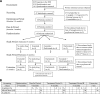
Figure 1
Study design (A) and timeline for testing procedures (B): 20 patients entered the optimization period, 16 were randomized, and 14 completed the entire trial. B: A, arginine stimulation test; M, mixed-meal test; T, T-cell proliferation test.
Figure 2
Results of C-peptide responses to arginine stimulation (A and B) and changes of insulin doses and weight according to treatment assignment (C). C-peptide results are shown at screening (basal versus stimulated C-peptide, P < 0.0001) and during run-in period (tests were conducted in months 2, 3, and 4 of run-in period [basal versus stimulated C-peptide, P = 0.0078]) (A) after 6 months of exenatide therapy versus not having received exenatide (irrespective of assignment to daclizumab) (B) with reference to mean results of run-in period. Data are means ± SEM. To convert C-peptide from conventional (ng/ml) to _S_i units (nmol/l), multiply by factor 0.333.
Figure 3
Results of mixed-meal testing, conducted according to study timeline (Fig. 1_B_). Exenatide administered before a mixed meal delayed gastric emptying (A; P = 0.041) and glucose absorption (B; P = 0.052), did not change glucagon levels (C; P = 0.414), and suppressed GLP-1 (D; P = 0.024). P values reflect differences of areas under the curve comparing patients' results on and off exenatide; data are means ± SEM. To convert glucose from conventional (mg/dl) to _S_i units (nmol/l), multiply by factor 0.0555.
References
- Hirshberg B, Rother KI, Digon BJ, 3rd, Lee J, Gaglia JL, Hines K, Read EJ, Chang R, Wood BJ, Harlan DM: Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care 2003; 26: 3288– 3295 - PubMed
- Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41– 46 - PubMed
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007; 12: 817– 826 - PubMed
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763– 1769 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical