ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis - PubMed (original) (raw)
ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis
Ozlem Yilmaz et al. Cell Microbiol. 2010 Feb.
Abstract
Production of IL-1beta typically requires two-separate signals. The first signal, from a pathogen-associated molecular pattern, promotes intracellular production of immature cytokine. The second signal, derived from a danger signal such as extracellular ATP, results in assembly of an inflammasome, activation of caspase-1 and secretion of mature cytokine. The inflammasome component, Nalp3, plays a non-redundant role in caspase-1 activation in response to ATP binding to P2X(7) in macrophages. Gingival epithelial cells (GECs) are an important component of the innate-immune response to periodontal bacteria. We had shown that GECs express a functional P2X(7) receptor, but the ability of GECs to secrete IL-1beta during infection remained unknown. We find that GECs express a functional Nalp3 inflammasome. Treatment of GECs with LPS or infection with the periodontal pathogen, Porphyromonas gingivalis, induced expression of the il-1beta gene and intracellular accumulation of IL-1beta protein. However, IL-1beta was not secreted unless LPS-treated or infected cells were subsequently stimulated with ATP. Conversely, caspase-1 is activated in GECs following ATP treatment but not P. gingivalis infection. Furthermore, depletion of Nalp3 by siRNA abrogated the ability of ATP to induce IL-1beta secretion in infected cells. The Nalp3 inflammasome is therefore likely to be an important mediator of the inflammatory response in gingival epithelium.
Figures
Figure 1
Primary GECs express mRNA for NLR family members and P. gingivalis infection stimulates the il-1β gene transcription. (A) il-1β gene expression was measured by quantitative, real time PCR (qPCR) from GECs infected with P. gingivalis at an MOI = 100 for 6 hrs, and uninfected (control). GAPDH was included as an internal control. Fold change was calculated by the comparative cycle threshold method. P < 0.001 for cells uninfected, control (*), compared with cells infected with P. gingivalis (**). Data are representative of two independent experiments performed in duplicates. (B) PCR amplification was carried out with primers specific for Nalp3, Nalp1 and Ipaf, as described in Experimental Procedures. An amplicon of the expected size (200 bp) was found for Nalp3. The control, without primers (NTC), did not produce a detectable band.
Figure 1
Primary GECs express mRNA for NLR family members and P. gingivalis infection stimulates the il-1β gene transcription. (A) il-1β gene expression was measured by quantitative, real time PCR (qPCR) from GECs infected with P. gingivalis at an MOI = 100 for 6 hrs, and uninfected (control). GAPDH was included as an internal control. Fold change was calculated by the comparative cycle threshold method. P < 0.001 for cells uninfected, control (*), compared with cells infected with P. gingivalis (**). Data are representative of two independent experiments performed in duplicates. (B) PCR amplification was carried out with primers specific for Nalp3, Nalp1 and Ipaf, as described in Experimental Procedures. An amplicon of the expected size (200 bp) was found for Nalp3. The control, without primers (NTC), did not produce a detectable band.
Figure 2
Primary GECs express a functional inflammasome. IL-1β secretion was measured in supernatants from GECs incubated with control buffer (Ctrl), incubated with 2 μg/ml of E. coli LPS for 6 hrs, infected with P. gingivalis (P.g.) at an MOI = 100 for 6 hrs, incubated with 5 mM ATP for 3 hrs, or infected with P. gingivalis for 6 hrs and then incubated for an additional 3 hrs with 5 mM ATP. IL-1β concentrations were quantified with an IL-1β ELISA kit. The values show averages and S.D. from 3 samples of a representative experiment, and represent results obtained from at least three experiments. P < 0.05 for cells infected with P. gingivalis and cells treated with ATP (*), compared with cells infected with P. gingivalis and then incubated with ATP (**).
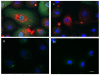
Figure 3
Cytokine is produced intracellularly during P. gingivalis infection but is released from cells following ATP treatment. (A) Intracellular cytokine (pro-IL-1β and IL-1β) was detected by immunofluorescence using antibodies against IL-1β (green). The samples were also stained with P. gingivalis antibody (red) and DAPI (blue) to visualize the nuclei. (i) There is a large increase in IL-1β staining in GECs infected with P. gingivalis at an MOI = 100 for 6 hrs. (ii) Most cytokine is released from cells that had been infected with P. gingivalis for 6 hrs and then incubated with 5 mM ATP for an additional 3 hrs. (iii) There is very little cytokine in GECs incubated with 5 mM ATP (iiii) or control buffer for 3 hrs. The images are representative of 150 cells studied per sample from at least two separate experiments performed in duplicate. Bar 10 μm. (B) Images of cells prepared as in Fig. 3A were captured with a fluorescence microscope equipped with a cooled CCD. The levels of intracellular cytokine (pro-IL-1β and IL-1β) in cells incubated with control buffer (Ctrl), treated with 5 mM ATP for 3 hrs, infected with P. gingivalis at an MOI = 100 for 6 hrs (P.g.), or infected with P. gingivalis for 6 hrs and then treated with ATP for 3 hrs, were quantified by measuring relative fluorescence with NIH ImageJ analysis software, as described in Experimental Procedures. Data were expressed as a percentage of the fluorescence intensity of the control samples. Results are representative images of 150 cells studied per sample from at least two individual experiments performed in duplicate. The values show averages and S.D. from a representative experiment. P = 0.05 for cells infected with P. gingivalis, compared with cells infected with P. gingivalis and then incubated with ATP.
Figure 3
Cytokine is produced intracellularly during P. gingivalis infection but is released from cells following ATP treatment. (A) Intracellular cytokine (pro-IL-1β and IL-1β) was detected by immunofluorescence using antibodies against IL-1β (green). The samples were also stained with P. gingivalis antibody (red) and DAPI (blue) to visualize the nuclei. (i) There is a large increase in IL-1β staining in GECs infected with P. gingivalis at an MOI = 100 for 6 hrs. (ii) Most cytokine is released from cells that had been infected with P. gingivalis for 6 hrs and then incubated with 5 mM ATP for an additional 3 hrs. (iii) There is very little cytokine in GECs incubated with 5 mM ATP (iiii) or control buffer for 3 hrs. The images are representative of 150 cells studied per sample from at least two separate experiments performed in duplicate. Bar 10 μm. (B) Images of cells prepared as in Fig. 3A were captured with a fluorescence microscope equipped with a cooled CCD. The levels of intracellular cytokine (pro-IL-1β and IL-1β) in cells incubated with control buffer (Ctrl), treated with 5 mM ATP for 3 hrs, infected with P. gingivalis at an MOI = 100 for 6 hrs (P.g.), or infected with P. gingivalis for 6 hrs and then treated with ATP for 3 hrs, were quantified by measuring relative fluorescence with NIH ImageJ analysis software, as described in Experimental Procedures. Data were expressed as a percentage of the fluorescence intensity of the control samples. Results are representative images of 150 cells studied per sample from at least two individual experiments performed in duplicate. The values show averages and S.D. from a representative experiment. P = 0.05 for cells infected with P. gingivalis, compared with cells infected with P. gingivalis and then incubated with ATP.
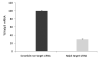
Figure 4
Depletion of Nalp3 abrogates the ability of ATP to induce IL-1β secretion from _P. gingivalis_-infected GECs. (A) Nalp3 gene expression was transiently silenced in primary by siRNA transfection, as described in Experimental Procedures. The expression levels for each condition were analyzed by qRT-PCR using the Ct method, and were represented as percentages of the non-target siRNA sample normalized to 100. The knockdown experiments were repeated at least three separate times demonstrating consistently ~ 70% decrease in the Nalp3 gene transcript in each time. (B) Primary GECs were treated with Nalp3 or non-target siRNA reagents, and IL-1β secretion from Nalp3 knockdowns (KD) and controls was measured by ELISA. The cells infected with P. gingivalis (P.g.) with or without transfection with siRNA (Nalp3 KD) did not produce any noticeable IL-1β secretion. On the other hand, depletion of Nalp3 in cells infected with P. gingivalis and then incubated with ATP (Nalp3 KD + P.g. + ATP) resulted in significant inhibition in secretion of IL-1β, compared with GECs that were not treated with siRNA (P.g. + ATP). The values show averages and S.D. from duplicate samples of a representative experiment, and show results obtained from at least 2 separate experiments. P = 0.05 for cells infected with P. gingivalis and then incubated with ATP (P.g. + ATP *), compared with cells that were transfected with Nalp3 siRNA, infected with P. gingivalis, and then incubated with ATP (P.g. + ATP, Nalp3 KD **).
Figure 4
Depletion of Nalp3 abrogates the ability of ATP to induce IL-1β secretion from _P. gingivalis_-infected GECs. (A) Nalp3 gene expression was transiently silenced in primary by siRNA transfection, as described in Experimental Procedures. The expression levels for each condition were analyzed by qRT-PCR using the Ct method, and were represented as percentages of the non-target siRNA sample normalized to 100. The knockdown experiments were repeated at least three separate times demonstrating consistently ~ 70% decrease in the Nalp3 gene transcript in each time. (B) Primary GECs were treated with Nalp3 or non-target siRNA reagents, and IL-1β secretion from Nalp3 knockdowns (KD) and controls was measured by ELISA. The cells infected with P. gingivalis (P.g.) with or without transfection with siRNA (Nalp3 KD) did not produce any noticeable IL-1β secretion. On the other hand, depletion of Nalp3 in cells infected with P. gingivalis and then incubated with ATP (Nalp3 KD + P.g. + ATP) resulted in significant inhibition in secretion of IL-1β, compared with GECs that were not treated with siRNA (P.g. + ATP). The values show averages and S.D. from duplicate samples of a representative experiment, and show results obtained from at least 2 separate experiments. P = 0.05 for cells infected with P. gingivalis and then incubated with ATP (P.g. + ATP *), compared with cells that were transfected with Nalp3 siRNA, infected with P. gingivalis, and then incubated with ATP (P.g. + ATP, Nalp3 KD **).
Figure 5
Stimulation with ATP activates caspase-1 in GECs. Primary GECs were infected with P. gingivalis at an MOI = 100 for 6 hrs (P.g.), treated with 2 μg/ml of E. coli LPS for 6 hrs, incubated with 5 mM ATP for 3 hrs, or incubated with 5 mM ATP for 3 hrs after 6 hrs of infection or LPS-treatment. Caspase-1 activation was measured by incubating treated or infected cells with a fluorescent caspase-1 substrate, as described in Experimental Procedures. LPS treatment or P. gingivalis infection have no effect on caspase-1 activity, but subsequent treatment with ATP induces a large increase in caspase-1 activation. The values show percent averages and S.D. from 3 samples of a representative experiment, and represent results obtained from at least three experiments. P = 0.02 for cells infected with P. gingivalis, compared with cells infected with P. gingivalis and then incubated with ATP.
Figure 6
The gene expression levels of Nalp3 but not ASC decreased partially after P. gingivalis infection. GECs were uninfected and untreated (control), infected with P. gingivalis at an MOI of 100 for 6 hrs, or infected and treated for an additional 3 hrs with 5 mM ATP. The expression levels of Nalp3 and ASC were compared with GAPDH and normalized to uninfected GEC responses, as described in Experimental Procedures. Data are representative of two independent experiments performed in duplicates.
Similar articles
- P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation.
Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz Ö, Ojcius DM. Hung SC, et al. PLoS One. 2013 Jul 25;8(7):e70210. doi: 10.1371/journal.pone.0070210. Print 2013. PLoS One. 2013. PMID: 23936165 Free PMC article. - Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.
Wang PL, Ohura K. Wang PL, et al. Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review. - Historical aspects of studies on roles of the inflammasome in the pathogenesis of periodontal diseases.
Shibata K. Shibata K. Mol Oral Microbiol. 2018 Jun;33(3):203-211. doi: 10.1111/omi.12217. Epub 2018 Feb 20. Mol Oral Microbiol. 2018. PMID: 29360244 Review.
Cited by
- Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase.
Johnson L, Atanasova KR, Bui PQ, Lee J, Hung SC, Yilmaz Ö, Ojcius DM. Johnson L, et al. Microbes Infect. 2015 May;17(5):369-77. doi: 10.1016/j.micinf.2015.03.010. Epub 2015 Mar 27. Microbes Infect. 2015. PMID: 25828169 Free PMC article. - Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines.
Shenker BJ, Ojcius DM, Walker LP, Zekavat A, Scuron MD, Boesze-Battaglia K. Shenker BJ, et al. Infect Immun. 2015 Apr;83(4):1487-96. doi: 10.1128/IAI.03132-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644004 Free PMC article. - Prelude to oral microbes and chronic diseases: past, present and future.
Atanasova KR, Yilmaz Ö. Atanasova KR, et al. Microbes Infect. 2015 Jul;17(7):473-83. doi: 10.1016/j.micinf.2015.03.007. Epub 2015 Mar 24. Microbes Infect. 2015. PMID: 25813714 Free PMC article. - Sorghum Phenolics Inhibits Inflammasomes in Lipopolysaccharide (LPS)-Primed and Adenosine Triphosphate (ATP)-Activated Macrophages.
Dia VP, Bradwell J, Pangloli P. Dia VP, et al. Plant Foods Hum Nutr. 2019 Sep;74(3):307-315. doi: 10.1007/s11130-019-00736-8. Plant Foods Hum Nutr. 2019. PMID: 31104201 - Breaking bad: manipulation of the host response by Porphyromonas gingivalis.
Hajishengallis G, Lamont RJ. Hajishengallis G, et al. Eur J Immunol. 2014 Feb;44(2):328-38. doi: 10.1002/eji.201344202. Eur J Immunol. 2014. PMID: 24338806 Free PMC article. Review.
References
- Agostini L, Martinon F, Burns K, McDermott MF, P.N. H, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
- Akman A, Ekinci NC, Kacaroglu H, Yavuzer U, Alpsoy E, Yegin O. Relationship between periodontal findings and specific polymorphisms of interleukin1alpha and -1beta in Turkish patients with Behçet’s disease. Arch. Dermatol. Res. 2008;300:19–26. - PubMed
- Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J. Biol. Chem. 2004;279:51897–51907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE016593-04/DE/NIDCR NIH HHS/United States
- R01 DE019444/DE/NIDCR NIH HHS/United States
- R01DE019444/DE/NIDCR NIH HHS/United States
- R01 DE016593/DE/NIDCR NIH HHS/United States
- R01DE016593/DE/NIDCR NIH HHS/United States
- R56 DE016593/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources