Regulation of TRPC1 and TRPC4 cation channels requires an alpha1-syntrophin-dependent complex in skeletal mouse myotubes - PubMed (original) (raw)
Regulation of TRPC1 and TRPC4 cation channels requires an alpha1-syntrophin-dependent complex in skeletal mouse myotubes
Jessica Sabourin et al. J Biol Chem. 2009.
Abstract
The dystrophin-associated protein complex (DAPC) is essential for skeletal muscle, and the lack of dystrophin in Duchenne muscular dystrophy results in a reduction of DAPC components such as syntrophins and in fiber necrosis. By anchoring various molecules, the syntrophins may confer a role in cell signaling to the DAPC. Calcium disorders and abnormally elevated cation influx in dystrophic muscle cells have suggested that the DAPC regulates some sarcolemmal cationic channels. We demonstrated previously that mini-dystrophin and alpha1-syntrophin restore normal cation entry in dystrophin-deficient myotubes and that sarcolemmal TRPC1 channels associate with dystrophin and the bound PDZ domain of alpha1-syntrophin. This study shows that small interfering RNA (siRNA) silencing of alpha1-syntrophin dysregulated cation influx in myotubes. Moreover, deletion of the PDZ-containing domain prevented restoration of normal cation entry by alpha1-syntrophin transfection in dystrophin-deficient myotubes. TRPC1 and TRPC4 channels are expressed at the sarcolemma of muscle cells; forced expression or siRNA silencing showed that cation influx regulated by alpha1-syntrophin is supported by TRPC1 and TRPC4. A molecular association was found between TRPC1 and TRPC4 channels and the alpha1-syntrophin-dystrophin complex. TRPC1 and TRPC4 channels may form sarcolemmal channels anchored to the DAPC, and alpha1-syntrophin is necessary to maintain the normal regulation of TRPC-supported cation entry in skeletal muscle. Cation channels with DAPC form a signaling complex that modulates cation entry and may be crucial for normal calcium homeostasis in skeletal muscles.
Figures
FIGURE 1.
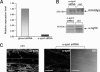
Extinction of α1-syntrophin transcript and reduction of α1-syntrophin protein in minidys+ SolD6 myotubes. A, 3-day differentiated minidys+ SolD6 muscle cells were harvested after transfection with siRNA against α1-syntrophin. Bar graphs represent the amount of α1-syntrophin mRNA analyzed by quantitative real-time PCR (value relative to the siRNA ghost control oligo). B, expression levels of α1-syntrophin in minidys+ SolD6 myotube control cells (ctrl) or in cells transfected with α1-syntrophin siRNA were analyzed by Western blots using an α1-syntrophin polyclonal antibody. The level of α1-syntrophin was compared with that of mini-dystrophin detected with a polyclonal antibody. C, immunofluorescence microscopy of minidys+ SolD6 myotubes transfected with α1-syntrophin siRNA. The right panel represents a phase contrast picture of the same α1-syntrophin siRNA-transfected cells in the central panel. Bars = 10 μ
m
.
FIGURE 2.
α1-Syntrophin is essential for normal regulation of cation entry in cultured myotubes. A, protein lysates from minidys+ SolD6 myotubes transfected with siRNA against α1-syntrophin or from untransfected cells (control (ctrl)) were incubated with polyclonal antibody raised against α1-syntrophin (α_-synt lane_). Western blots of the immunoprecipitated proteins (IP) were probed with a polyclonal antibody against mini-dystrophin and a polyclonal antibody against α1-syntrophin. B, protein lysates from minidys+ SolD6 myotubes transfected with siRNA against α1-syntrophin or from untransfected cells were incubated with polyclonal antibody raised against TRPC1 and α1-syntrophin. Western blots of the immunoprecipitated proteins were probed with a polyclonal antibody against α1-syntrophin and a polyclonal antibody against TRPC1. C, three representative recordings of fura-2 fluorescence during perfusion of 50 μ
m
Mn2+ obtained from nontransfected minidys+ SolD6 myotubes (dotted line), from a minidys+ SolD6 myotubes transfected with siRNA against α1-syntrophin (bolder gray line), and from a dystrophin-deficient SolC1 myotubes (gray line). D, slopes of the Mn2+-induced decreasing phase of fura-2 fluorescence were measured and expressed as percent decrease/min. Bar graphs represent mean rates of fluorescence decrease induced by Mn2+ (expressed in %/min) ± S.E. in control SolD6 myotubes (black column), in control SolD6 myotubes transfected with siRNA ghost (dark gray column), and in SolD6 transfected with α1-syntrophin siRNA (light gray column). The white column represents abnormally elevated cation entry in dys− SolC1 myotubes. The difference between mean values of measured parameters was determined by Student's t test and considered significant at p < 0.05 (***, p < 0.001). The cationic influx measured under the dotted line represents the background cation entry insensitive to SKF-96365. n.s., no significant.
FIGURE 3.
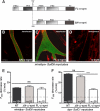
The N terminus corresponding to the PH1a and PDZ domains of α1-syntrophin is necessary for regulating the abnormally elevated cation entry in dystrophin-deficient cultured myotubes. A, structural diagram of FL-α1-syntrophin and α1-syntrophin missing the PH1a and PDZ domains (Δ_N_), showing the relative localization of the PH domains, the PDZ domain, and the syntrophin-unique domain. B–D, differentiated minidys+ SolD6 myotubes were transfected with plasmids encoding ΔN-α1-syntrophin-EGFP and FL-α1-syntrophin-EGFP. Myotubes were immunostained with antibody specific for α1-syntrophin (in red). EGFP expression in cytoplasm is shown in green. The expression level of α1-syntrophin was evaluated by the intensity of red fluorescence. Bars = 10 μ
m
. E and F, minidys+ SolD6 and dys− SolC1 myotubes were transfected with the expression vector encoding ΔN-α1-syntrophin-EGFP. E, mean rates of fluorescence decrease induced by Mn2+ (expressed in %/min) ± S.E. in minidys+ SolD6 myotubes transfected (gray column) with the ΔN-α1-syntrophin-EGFP or nontransfected (NT, black column). ns, not significant. F, same experiment as in E on dys− SolC1 myotubes. The white columns represent results of the effect of FL-α1-syntrophin forced expression on cation entry, which confirms those already reported by Vandebrouck et al. (7).
FIGURE 4.
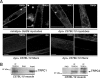
TRPC4 and TRPC1 are present at the sarcolemma of muscle and cultured myotubes and form a stable complex in skeletal muscle. A, laser scanning confocal microscopy analysis of TRPC1 and TRPC4 distribution. Immunostaining in minidys+ SolD6 cultured myotubes, in dys+ C57BL/10 myotubes from primary cultures, and in C57BL/10 skeletal muscle (semi-thin section) is shown. Costaining of dystrophin in skeletal muscle was obtained with a mouse monoclonal antibody to show the cortical compartment of muscle fibers. Bars = 10 μ
m
. B, muscle lysates were incubated with polyclonal antibodies against TRPC1 (IP: TRPC1 lane) or TRPC4 (IP: TRPC4 lane). Immunoprecipitation was carried out with protein A-Sepharose beads (PAS). Western blot of the immunoprecipitated proteins was probed with polyclonal antibodies against TRPC1 or TRPC4. Positive controls for immunoprecipitation were carried out with antibodies raised against TRPC1 or TRPC4, respectively, and negative controls were performed with protein A-Sepharose.
FIGURE 5.
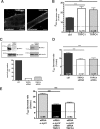
TRPC1 and TRPC4 are involved in cation influx regulated by α1-syntrophin in minidys+ SolD6 myotubes. A, analysis by laser scanning confocal microscopy of Myc-tagged TRPC4 and FLAG-tagged TRPC1 immunostaining in transfected minidys+ SolD6 cultured myotubes. Bars = 10 μ
m
. B, minidys+ SolD6 myotubes were transfected with the expression vector encoding TRPC1 or TRPC4. Mean rates of fluorescence decrease induced by Mn2+ (expressed in %/min) ± S.E. are shown in control minidys+ SolD6 myotubes (ctrl; black column) and in minidys+ SolD6 transfected with TRPC1 (white column) and TRPC4 (gray column). The difference between mean values of measured parameters was processed by Student's t test and considered significant at p < 0.05 (***, p < 0.001). C, histograms show efficiency of TRPC1 and TRPC4 expression after siRNA treatment. TRPC1 and TRPC4 protein levels were normalized to α-tubulin and expressed as percent of control. D, effect of siRNA knockdown of individual TRPC homologues on store-operated cation entry. Minidys+ SolD6 were transfected with TRPC1 or TRPC4 siRNA. Mean rates of fluorescence decrease induced by Mn2+ (expressed in %/min) ± S.E. are shown in control minidys+ SolD6 myotubes (black column) and in minidys+ SolD6 transfected TRPC1 siRNA (white column) and TRPC4 siRNA (gray column). The difference between mean values of measured parameters was determined by Student's t test and was considered significant at p < 0.05 (***, p < 0.001). E, effect of double siRNA knockdown of α1-syntrophin and TRPC1 and, on the other hand, of α1-syntrophin and TRPC4 on store-operated cation influx in minidys+ SolD6 myotubes. Mean rates of fluorescence decrease induced by Mn2+ (expressed in %/min) ± S.E. are shown in minidys+ SolD6 myotubes transfected with α1-syntrophin siRNA (gray column) and in minidys+ SolD6 transfected with double siRNAs against α1-syntrophin/TRPC1 (black column) and double siRNAs against α1-syntrophin/TRPC4 (white column). The difference between mean values of measured parameters was analyzed by Student's t test and considered significant at p < 0.05 (***, p < 0.001).
FIGURE 6.
TRPC4 associates in vitro with the PDZ domain of α1-syntrophin and forms a macromolecular complex with TRPC1, dystrophin, and α1-syntrophin in skeletal muscle and cultured myotubes. A, the lysates from differentiated minidys+ SolD6 myotubes transfected with FL-α1-syntrophin-c-Myc-EGFP plasmid were immunoprecipitated (IP) with polyclonal antibodies against c-Myc tag (c-Mycp lane), TRPC4 (TRPC4 lane), and dystrophin (dys lane). The resulting immunoblot (IB) was probed with a monoclonal antibody against c-Myc tag (c-Mycm). B, beads charged with GST alone or with a GST fusion protein of the PDZ domain of α1-syntrophin were incubated with protein lysates from minidys+ SolD6 myotubes. Bound proteins were immunoblotted with a polyclonal TRPC4 antibody or with a polyclonal TRPC1 antibody. The input lane was loaded with 10% of the extract used for the pulldown. C, muscle lysates from C57BL/10 muscles (input lane) were incubated with polyclonal antibodies against dystrophin (dys lane), α1-syntrophin (α_-synt lane_), TRPC1 (TRPC1 lane), and TRPC4 (TRPC4 lane). Western blots of the immunoprecipitated proteins were probed with polyclonal antibodies against TRPC4 or TRPC1. PAS, protein A-Sepharose. D, protein lysates (input lane) from minidys+ SolD6 myotubes were incubated with polyclonal antibodies against α1-syntrophin, TRPC4, and mini-dystrophin (dys lane). Western blots of the immunoprecipitated proteins were probed with polyclonal antibodies against α1-syntrophin or mini-dystrophin.
Similar articles
- Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin.
Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Vandebrouck A, et al. FASEB J. 2007 Feb;21(2):608-17. doi: 10.1096/fj.06-6683com. Epub 2007 Jan 3. FASEB J. 2007. PMID: 17202249 - Dystrophin/α1-syntrophin scaffold regulated PLC/PKC-dependent store-operated calcium entry in myotubes.
Sabourin J, Harisseh R, Harnois T, Magaud C, Bourmeyster N, Déliot N, Constantin B. Sabourin J, et al. Cell Calcium. 2012 Dec;52(6):445-56. doi: 10.1016/j.ceca.2012.08.003. Epub 2012 Aug 28. Cell Calcium. 2012. PMID: 22938798 - Involvement of TRPV2 and SOCE in calcium influx disorder in DMD primary human myotubes with a specific contribution of α1-syntrophin and PLC/PKC in SOCE regulation.
Harisseh R, Chatelier A, Magaud C, Déliot N, Constantin B. Harisseh R, et al. Am J Physiol Cell Physiol. 2013 May 1;304(9):C881-94. doi: 10.1152/ajpcell.00182.2012. Epub 2013 Feb 20. Am J Physiol Cell Physiol. 2013. PMID: 23426965 - Regulation by scaffolding proteins of canonical transient receptor potential channels in striated muscle.
Sabourin J, Cognard C, Constantin B. Sabourin J, et al. J Muscle Res Cell Motil. 2009 Dec;30(7-8):289-97. doi: 10.1007/s10974-010-9206-9. Epub 2010 Mar 2. J Muscle Res Cell Motil. 2009. PMID: 20195709 Review. - How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes.
Alderton JM, Steinhardt RA. Alderton JM, et al. Trends Cardiovasc Med. 2000 Aug;10(6):268-72. doi: 10.1016/s1050-1738(00)00075-x. Trends Cardiovasc Med. 2000. PMID: 11282306 Review.
Cited by
- Ion Channels of the Sarcolemma and Intracellular Organelles in Duchenne Muscular Dystrophy: A Role in the Dysregulation of Ion Homeostasis and a Possible Target for Therapy.
Dubinin MV, Belosludtsev KN. Dubinin MV, et al. Int J Mol Sci. 2023 Jan 23;24(3):2229. doi: 10.3390/ijms24032229. Int J Mol Sci. 2023. PMID: 36768550 Free PMC article. Review. - Biochemical and Functional Interplay Between Ion Channels and the Components of the Dystrophin-Associated Glycoprotein Complex.
Leyva-Leyva M, Sandoval A, Felix R, González-Ramírez R. Leyva-Leyva M, et al. J Membr Biol. 2018 Aug;251(4):535-550. doi: 10.1007/s00232-018-0036-9. Epub 2018 May 19. J Membr Biol. 2018. PMID: 29779049 Review. - LQTS-associated mutation A257G in α1-syntrophin interacts with the intragenic variant P74L to modify its biophysical phenotype.
Cheng J, Norstrand DW, Medeiros-Domingo A, Tester DJ, Valdivia CR, Tan BH, Vatta M, Makielski JC, Ackerman MJ. Cheng J, et al. Cardiogenetics. 2011 Oct 25;1(1):136. doi: 10.4081/cardiogenetics.2011.e13. Cardiogenetics. 2011. PMID: 24319568 Free PMC article. - Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy.
Ohlendieck K, Swandulla D. Ohlendieck K, et al. Pflugers Arch. 2021 Dec;473(12):1813-1839. doi: 10.1007/s00424-021-02623-1. Epub 2021 Sep 22. Pflugers Arch. 2021. PMID: 34553265 Free PMC article. Review. - The Dystrophin Node as Integrator of Cytoskeletal Organization, Lateral Force Transmission, Fiber Stability and Cellular Signaling in Skeletal Muscle.
Dowling P, Gargan S, Murphy S, Zweyer M, Sabir H, Swandulla D, Ohlendieck K. Dowling P, et al. Proteomes. 2021 Feb 2;9(1):9. doi: 10.3390/proteomes9010009. Proteomes. 2021. PMID: 33540575 Free PMC article. Review.
References
- Ervasti J. M., Campbell K. P. (1991) Cell 66, 1121–1131 - PubMed
- Compton A. G., Cooper S. T., Hill P. M., Yang N., Froehner S. C., North K. N. (2005) J. Neuropathol. Exp. Neurol. 64, 350–361 - PubMed
- Adams M. E., Butler M. H., Dwyer T. M., Peters M. F., Murnane A. A., Froehner S. C. (1993) Neuron 11, 531–540 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases