Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Dec 15;115(24):5828-35.
doi: 10.1002/cncr.24667.
Affiliations
- PMID: 19813275
- PMCID: PMC2795100
- DOI: 10.1002/cncr.24667
Randomized Controlled Trial
Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group
Howard M Katzenstein et al. Cancer. 2009.
Abstract
Background: The current study was conducted to determine whether amifostine is effective in reducing the toxicities associated with the administration of platinum-containing regimens in children with hepatoblastoma (HB).
Methods: Patients were enrolled on P9645 beginning in March of 1999. Patients who had stage I/II disease received treatment with 4 cycles of combined cisplatin, 5-fluorouracil, and vincristine (C5V) with or without amifostine. Patients who had stage III/IV disease were randomized to receive treatment with 6 cycles of either C5V with or without amifostine or carboplatin alternating with cisplatin (CC) with or without amifostine. Patients who were randomized to receive amifostine were given a dose of 740 mg/m2 intravenously over 15 minutes before each administration of a platinum agent.
Results: Eighty-two patients were considered in a special interim analysis of the incidence of toxicity. The disease outcome for patients who received amifostine was similar to the outcome for patients who did not receive amifostine (P=.22). The incidence of significant hearing loss (>40 dB) was similar for patients who did or did not receive amifostine (38% [14 of 37 patients] vs 38% [17 of 45 patients], respectively; P=.68). There were no differences in the incidence of renal or bone marrow toxicities evaluated. Patients who received amifostine had a higher incidence of hypocalcemia (5% vs 0.5%; P=.00006).
Conclusions: Amifostine in the doses and schedule used in this study failed to significantly reduce the incidence of platinum-induced toxicities in patients with HB.
Copyright (c) 2009 American Cancer Society.
Figures
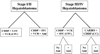
Figure 1
Treatment schema for patients on COG P9645. CDDP=cisplatin, 5-FU=5-fluorouracil, VCR=vincristine CARBO=carboplatin, AMI=amifostine
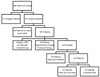
Figure 2
Evaluation cohort
Figure 3
EFS of 184 patients who enrolled before 3/31/2003 and received chemotherapy by stratum of amifostine randomization. Adjusted p-value for the risk of an adverse event associated with amifostine = 0.22.
Comment in
- Hepatoblastoma, cisplatin, and ototoxicity: good news on deaf ears.
Sullivan MJ. Sullivan MJ. Cancer. 2009 Dec 15;115(24):5623-6. doi: 10.1002/cncr.24668. Cancer. 2009. PMID: 19813276 No abstract available.
Similar articles
- Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD009219. doi: 10.1002/14651858.CD009219.pub3. Cochrane Database Syst Rev. 2014. PMID: 24984156 Review. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2012 May 16;(5):CD009219. doi: 10.1002/14651858.CD009219.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592737 Updated. Review. - Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma.
Malogolowkin MH, Katzenstein H, Krailo MD, Chen Z, Bowman L, Reynolds M, Finegold M, Greffe B, Rowland J, Newman K, Womer RB, London WB, Castleberry RP. Malogolowkin MH, et al. J Clin Oncol. 2006 Jun 20;24(18):2879-84. doi: 10.1200/JCO.2005.02.6013. J Clin Oncol. 2006. PMID: 16782927 Clinical Trial. - [Differentiated treatment protocols for high- and standard-risk hepatoblastoma--an interim report of the German Liver Tumor Study HB99].
Häberle B, Bode U, von Schweinitz D. Häberle B, et al. Klin Padiatr. 2003 May-Jun;215(3):159-65. doi: 10.1055/s-2003-39375. Klin Padiatr. 2003. PMID: 12778356 Clinical Trial. German. - Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group.
Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, Haas JE, King DR, Liu-Mares W, Sensel MG, Krailo MD. Ortega JA, et al. J Clin Oncol. 2000 Jul;18(14):2665-75. doi: 10.1200/JCO.2000.18.14.2665. J Clin Oncol. 2000. PMID: 10894865 Clinical Trial.
Cited by
- Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline.
Freyer DR, Brock PR, Chang KW, Dupuis LL, Epelman S, Knight K, Mills D, Phillips R, Potter E, Risby D, Simpkin P, Sullivan M, Cabral S, Robinson PD, Sung L. Freyer DR, et al. Lancet Child Adolesc Health. 2020 Feb;4(2):141-150. doi: 10.1016/S2352-4642(19)30336-0. Epub 2019 Dec 19. Lancet Child Adolesc Health. 2020. PMID: 31866182 Free PMC article. Review. - Ototoxicity: A Challenge in Diagnosis and Treatment.
Ganesan P, Schmiedge J, Manchaiah V, Swapna S, Dhandayutham S, Kothandaraman PP. Ganesan P, et al. J Audiol Otol. 2018 Apr;22(2):59-68. doi: 10.7874/jao.2017.00360. Epub 2018 Feb 26. J Audiol Otol. 2018. PMID: 29471610 Free PMC article. Review. - Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss.
Hazlitt RA, Min J, Zuo J. Hazlitt RA, et al. J Med Chem. 2018 Jul 12;61(13):5512-5524. doi: 10.1021/acs.jmedchem.7b01653. Epub 2018 Feb 1. J Med Chem. 2018. PMID: 29361217 Free PMC article. Review. - Platinum-induced ototoxicity: a review of prevailing ototoxicity criteria.
Waissbluth S, Peleva E, Daniel SJ. Waissbluth S, et al. Eur Arch Otorhinolaryngol. 2017 Mar;274(3):1187-1196. doi: 10.1007/s00405-016-4117-z. Epub 2016 May 31. Eur Arch Otorhinolaryngol. 2017. PMID: 27245751 Review. - Assessment and Management of Platinum-Related Ototoxicity in Children Treated for Cancer.
Romano A, Capozza MA, Mastrangelo S, Maurizi P, Triarico S, Rolesi R, Attinà G, Fetoni AR, Ruggiero A. Romano A, et al. Cancers (Basel). 2020 May 17;12(5):1266. doi: 10.3390/cancers12051266. Cancers (Basel). 2020. PMID: 32429551 Free PMC article. Review.
References
- Douglass EC, Reynolds M, Finegold M, et al. Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1993;11:96–99. - PubMed
- Ortega JA, Krailo MD, Haas JE, et al. Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: a report from the Childrens Cancer Study Group. J Clin Oncol. 1991;9:2167–2176. - PubMed
- Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. - PubMed
- Katzenstein HM, London WB, Douglass EC, et al. Treatment of unresectable and metastatic hepatoblastoma: a pediatric oncology group phase II study. J Clin Oncol. 2002;20:3438–3444. - PubMed
- Dall'Igna P, Cecchetto G, Dominici C, et al. Carboplatin and doxorubicin (CARDOX) for nonmetastatic hepatoblastoma: a discouraging pilot study. Med Pediatr Oncol. 2001;36:332–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA98413/CA/NCI NIH HHS/United States
- U10 CA098543-06/CA/NCI NIH HHS/United States
- U10 CA029139-22/CA/NCI NIH HHS/United States
- U10 CA098413/CA/NCI NIH HHS/United States
- U10CA029139/CA/NCI NIH HHS/United States
- U10 CA029139/CA/NCI NIH HHS/United States
- U10 CA98543/CA/NCI NIH HHS/United States
- U10 CA098413-06/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous