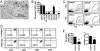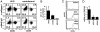CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes - PubMed (original) (raw)
CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes
Marianne Niedermeier et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Fibrocytes are collagen-type-I-producing cells that arise at low frequency from hematopoietic cells. We have analyzed in mice which leukocyte subsets are required for generation of fibrocytes and show that murine fibrocytes develop from the subpopulation of CD11b(+) CD115(+) Gr1(+) monocytes under the control of CD4(+) T cells. In the absence of CD4(+) T cells, differentiation of fibrocytes was markedly reduced in vitro and in vivo. In the presence of CD4(+) T cells, the characteristics of T-cell activation critically determined development of fibrocytes. Polyclonal activation of CD4(+) T cells induced the release of soluble factors that completely prevented the outgrowth of fibrocytes and could be identified as IL-2, TNF, IFN-gamma, and IL-4. Application of IL-2 and TNF significantly reduced the appearance of fibrocytes and the severity of fibrosis in the model of unilateral ureteral obstruction. In contrast, activation of CD4(+) T cells in the presence of calcineurin inhibitors, but not mTOR inhibitors, markedly enhanced the outgrowth of fibrocytes and renal deposition of collagen I. Taken together, we show that differentiation of fibrocytes is critically dependent on CD4(+) T cells and that the context of T-cell activation determines whether development of fibrocytes is supported or blocked. Our data may have implications for prevention of organ fibrosis in autoimmune diseases and transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Cellular origin of fibrocytes in mice. (A–E) Total splenocytes or splenocytes depleted of specific leukocyte subsets were cultured for 14 days. (A) Pictures of cultured splenocytes were taken under light microscopy. (B) Number of spindle-shaped cells was counted. Compared with culture of total splenocytes (spleen) or sham depletion (control), depletion of cells expressing CD4, CD16/32, CD11b, CD115, or Gr1 but not CD11c almost completely prevented the outgrowth of spindle-shaped cells. (C) Flow cytometric analysis of light scatter properties of splenocytes cultured after depletion of various leukocyte subpopulations. (D) Large cells located within gate R1 of C were analyzed for expression of CD45 and intracellular collagen I. Intracellular staining with an isotype control antibody (rabbit IgG) served as control. (E) Number of spindle-shaped cells per 105 splenocytes and quantification of collagen I in total cell lysates by ELISA after culture of sham-depleted splenocytes (control) or splenocytes depleted of CD11b+ cells. Results are expressed as the mean ± SEM of the number of spindle-shaped cells from three pictures of three wells/condition. Statistical significance has been determined in comparison with the control.
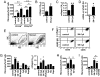
Fig. 2.
Conditions supporting the differentiation of monocytes isolated from splenocytes into fibrocytes. (A) CD11b+ or CD16/32+ monocytes were cultured for 14 days with medium (control), purified CD4+ T cells, or the supernatant of CD4+ T cells activated in the presence of cyclosporine A (SN CyA). Addition of CD4+ T cells or SN CyA markedly enhanced the appearance of spindle-shaped cells. (B–F) CD11b+ monocytes were incubated with medium or SN CyA. (B) Amount of intracellular collagen I in total cell lysates. (C) Amount of collagen I secreted onto the culture plate was measured by ELISA. (D) Expression of collagen I mRNA (col I) relative to GAPDH was quantified by real-time RT-PCR in cultured cells. (E and F) Light scatter properties of cultured cells (E) and expression of CD45 and intracellular collagen I compared with an isotype control (rabbit IgG) (F) were analyzed by flow cytometry. (G) CD11b+ monocytes were cultured with medium (control), with SN CyA, SN TAC, SN Rapa, or directly with cyclosporine A (CyA), tacrolimus (TAC), or rapamycin (Rapa). The number of spindle-shaped cells was counted, and the amount of secreted collagen I was measured by ELSIA. (H) Numbers of spindle-shaped cells after culture of CD11b+ monocytes with medium (control) SN CyA or boiled SN CyA (95 °C). (I) Numbers of spindle-shaped cells after culture of CD11b+ monocytes with isotype control antibody (control), anti-TGF-β, SN CyA or SN CyA preincubated with anti-TGF-β. Cell culture experiments were performed in triplicates. Results are expressed as the mean ± SEM of cell culture triplicates. Statistical significance has been determined in comparison with the medium control.
Fig. 3.
Influence of T-cell-derived cytokines on the generation of fibrocytes. (A and B) CD11b+ monocytes isolated from splenocytes were cultured for 14 days with medium (control), supernatant of CD4+ T cells activated in absence (SN) or in presence of cyclosporine A (SN CyA). (A) Addition of SN completely prevented, whereas addition of SN CyA markedly enhanced, the appearance of spindle-shaped cells. (B) Light scatter properties of cultured cells and their expression of CD45 and intracellular collagen I compared with an isotype control (rabbit IgG) were analyzed by flow cytometry. (C) CD11b+ monocytes were cultured with or without SN or SN preincubated with neutralizing antibodies against IL-2, IL-4, TNF, INFγ, and IL-21, as indicated. The number of spindle-shaped cells was counted. (D) CD11b+ monocytes were cultured for 14 days with the indicated cytokines. The numbers of spindle-shaped cells were counted, and secretion of collagen I to the culture plate was quantified by ELISA. Results are expressed as the mean ± SEM of the number of fibrocytes from three pictures of three wells/condition. Statistical significance was determined in comparison with medium control.
Fig. 4.
Detection of fibrocytes in the model of UUO. (A and B) On day 7 after induction of UUO in C57BL/6 mice, single-cell suspensions of the obstructed kidneys (UUO), the contralateral kidneys (contralateral), and the spleens were stained for surface expression of CD45 and intracellular collagen I or rabbit IgG as control and analyzed by flow cytometry. (A) Representative light scatter FACS-plots with the percentage of cells in each quadrant. (B) Number of CD45+ collagen I+ fibrocytes as percentage of total CD45+ cells in the obstructed kidneys, the contralateral kidneys, and the spleens (n = 5 per group). (C) Example of purification of CD45+ cells from UUO kidneys of rats at day 10 after UUO. Single-cell suspensions of UUO kidneys were analyzed for expression of CD45 before purification (before), after enrichment of CD45+ cells with beads (beads), and after further purification by FACS-sort (FACS). (D) Expression of collagen I mRNA (col I) relative to GAPDH in purified CD45+ cells from rat UUO kidneys (UUO), contralateral kidneys (contra), and spleens was quantified by real-time RT-PCR (n = 4 per group). Results are expressed as mean ± SEM. Statistical significance was determined compared with contralateral kidneys.
Fig. 5.
Modulation of fibrocyte differentiation in vivo. (A–E) Mice were treated from day 0 to 6 after induction of UUO with IL-2 and TNF or PBS as control (n = 5 per group). (A) Flow cytometric detection of CD45+ collagen I+ cells in UUO kidneys of mice treated with PBS (control) or IL-2 plus TNF. Staining with rabbit IgG served as control. (B) Number of CD45+ collagen I+ cells as percentage of infiltrating total CD45+ cells and (C) expression of collagen I mRNA (col I) relative to GAPDH in UUO kidneys (UUO), contralateral kidneys (contra), and spleens of mice treated with PBS (black bars) or IL-2 plus TNF (white bars). (D) Immunofluorescence of collagen I (red) and quantification of the collagen I positive area in sections of UUO kidneys of mice treated with IL-2 plus TNF or PBS (control). (E) Quantification of collagen I protein relative to GAPDH protein in UUO kidneys of IL-2 plus TNF or PBS-treated mice by Western blot analysis. (F) Quantification of CD45+ collagen I+ cells as percentage of infiltrating CD45+ cells by flow cytometry in UUO and contralateral kidneys (contra) of T-cell-deficient SCID mice and BALB/c control mice. (G–I) Renal fibrocytes in mice treated from day 0 to 6 after induction of UUO with αCD3 together with cyclosporine A (CyA), rapamycin (Rapa), or olive oil as control (oil) (n = 5 per group). Number of CD45+ collagen I+ cells as percentage of infiltrating CD45+ cells (G), expression of collagen I mRNA (col I) relative to GAPDH (H), and immunofluorescence of collagen I (red) (I) and quantification of the collagen I positive area in sections of UUO kidneys of various treatment groups.
Similar articles
- Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model.
Reich B, Schmidbauer K, Rodriguez Gomez M, Johannes Hermann F, Göbel N, Brühl H, Ketelsen I, Talke Y, Mack M. Reich B, et al. Kidney Int. 2013 Jul;84(1):78-89. doi: 10.1038/ki.2013.84. Epub 2013 Mar 13. Kidney Int. 2013. PMID: 23486523 - Depletion of CD8+ T Cells Exacerbates CD4+ T Cell-Induced Monocyte-to-Fibroblast Transition in Renal Fibrosis.
Dong Y, Yang M, Zhang J, Peng X, Cheng J, Cui T, Du J. Dong Y, et al. J Immunol. 2016 Feb 15;196(4):1874-81. doi: 10.4049/jimmunol.1501232. Epub 2016 Jan 15. J Immunol. 2016. PMID: 26773152 - Resting T cells negatively regulate osteoclast generation from peripheral blood monocytes.
Shinoda K, Sugiyama E, Taki H, Harada S, Mino T, Maruyama M, Kobayashi M. Shinoda K, et al. Bone. 2003 Oct;33(4):711-20. doi: 10.1016/s8756-3282(03)00230-8. Bone. 2003. PMID: 14555277 - Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis.
Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Sakai N, et al. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14098-103. doi: 10.1073/pnas.0511200103. Epub 2006 Sep 11. Proc Natl Acad Sci U S A. 2006. PMID: 16966615 Free PMC article. - Fibrocytes: A Critical Review and Practical Guide.
Reinhardt JW, Breuer CK. Reinhardt JW, et al. Front Immunol. 2021 Dec 17;12:784401. doi: 10.3389/fimmu.2021.784401. eCollection 2021. Front Immunol. 2021. PMID: 34975874 Free PMC article. Review.
Cited by
- Therapeutic IGF-I receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy.
Fernando R, Caldera O, Smith TJ. Fernando R, et al. Proc Natl Acad Sci U S A. 2021 Dec 28;118(52):e2114244118. doi: 10.1073/pnas.2114244118. Proc Natl Acad Sci U S A. 2021. PMID: 34949642 Free PMC article. - Here, There, and Everywhere: Myeloid-Derived Suppressor Cells in Immunology.
Ostrand-Rosenberg S, Lamb TJ, Pawelec G. Ostrand-Rosenberg S, et al. J Immunol. 2023 May 1;210(9):1183-1197. doi: 10.4049/jimmunol.2200914. J Immunol. 2023. PMID: 37068300 Free PMC article. Review. - Fibrocyte enrichment and myofibroblastic adaptation causes nucleus pulposus fibrosis and associates with disc degeneration severity.
Sun Y, Peng Y, Su Z, So KKH, Lu Q, Lyu M, Zuo J, Huang Y, Guan Z, Cheung KMC, Zheng Z, Zhang X, Leung VYL. Sun Y, et al. Bone Res. 2025 Jan 20;13(1):10. doi: 10.1038/s41413-024-00372-2. Bone Res. 2025. PMID: 39828732 Free PMC article. - Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments.
Lech M, Gröbmayr R, Weidenbusch M, Anders HJ. Lech M, et al. Mediators Inflamm. 2012;2012:951390. doi: 10.1155/2012/951390. Epub 2012 Dec 3. Mediators Inflamm. 2012. PMID: 23251037 Free PMC article. Review. - Fibrocytes in Fibrotic Diseases and Wound Healing.
Grieb G, Bucala R. Grieb G, et al. Adv Wound Care (New Rochelle). 2012 Feb;1(1):36-40. doi: 10.1089/wound.2011.0310. Adv Wound Care (New Rochelle). 2012. PMID: 24527276 Free PMC article. Review.
References
- Strutz F, Muller GA. Transdifferentiation comes of age. Nephrol Dial Transplant. 2000;15:1729–1731. - PubMed
- Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous