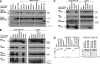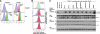TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis - PubMed (original) (raw)
. 2009 Dec 18;284(51):35906-15.
doi: 10.1074/jbc.M109.072256.
Delara Pantaki, Rebecca Feltham, Peter D Mace, Stephanie M Cordier, Anna C Schmukle, Angelina J Davidson, Bernard A Callus, Wendy Wei-Lynn Wong, Ian E Gentle, Holly Carter, Erinna F Lee, Henning Walczak, Catherine L Day, David L Vaux, John Silke
Affiliations
- PMID: 19815541
- PMCID: PMC2791019
- DOI: 10.1074/jbc.M109.072256
TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis
James E Vince et al. J Biol Chem. 2009.
Abstract
Tumor necrosis factor (TNF) receptor-associated factor-2 (TRAF2) binds to cIAP1 and cIAP2 (cIAP1/2) and recruits them to the cytoplasmic domain of several members of the TNF receptor (TNFR) superfamily, including the TNF-TNFR1 ligand-receptor complex. Here, we define a cIAP1/2-interacting motif (CIM) within the TRAF-N domain of TRAF2, and we use TRAF2 CIM mutants to determine the role of TRAF2 and cIAP1/2 individually, and the TRAF2-cIAP1/2 interaction, in TNFR1-dependent signaling. We show that both the TRAF2 RING domain and the TRAF2 CIM are required to regulate NF-kappaB-inducing kinase stability and suppress constitutive noncanonical NF-kappaB activation. Conversely, following TNFR1 stimulation, cells bearing a CIM-mutated TRAF2 showed reduced canonical NF-kappaB activation and TNF-induced RIPK1 ubiquitylation. Remarkably, the RING domain of TRAF2 was dispensable for these functions. However, like the TRAF2 CIM, the RING domain of TRAF2 was required for protection against TNF-induced apoptosis. These results show that TRAF2 has anti-apoptotic signaling roles in addition to promoting NF-kappaB signaling and that efficient activation of NF-kappaB by TNFR1 requires the recruitment of cIAP1/2 by TRAF2.
Figures
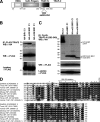
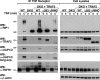
FIGURE 1.
Identification of a TRAF2-CIM. A, schematic of TRAF2 domain structure and localization of the CIM. aa, amino acids. B and C, TRAF-N domain of TRAF2 interacts with the BIR1 domain of cIAP1. 293T cells were co-transfected with the indicated HA-cIAP1 constructs and FLAG-TRAF2 (B) or FLAG-TRAF2-N (C), and their interaction was examined by FLAG immunoprecipitation (IP) and Western blot (WB). m22 and m39 refer to BIR1 deletion constructs lacking the first 22 and 39 residues, respectively. D, conservation within the TRAF2-N domain. The TRAF-N domain of TRAF2 was aligned from the indicated organisms (accession numbers in parentheses) and compared with the same region of human TRAF1 (bottom line). Identical residues are shaded in black and conserved residues in gray. Residues mutated in TRAF2 to examine the effect on cIAP1/2 binding are highlighted with an asterisk, and the deletion (283–293) and truncation mutants (Asp-310-stop) are also indicated.
FIGURE 2.
TRAF2 residues 292EVE294 and 283–293 are important for cIAP1 and cIAP2 binding to TRAF2. A, determination of TRAF2 residues required for cIAP1 binding. 293T cells were transfected with the indicated FLAG-TRAF2 constructs for 48 h and then immunoprecipitated (IP) from cell lysates. Binding of endogenous (endog.) cIAP1 to FLAG-TRAF2 was determined by Western blot (WB). The asterisk indicates IgG light chain cross-reactivity. B, mutation of both TRAF2 Glu-292 and Glu-294 is required to reduce cIAP1 and cIAP2 binding. 293T cells were transfected with the indicated FLAG-TRAF2 constructs in addition to cIAP2. FLAG-TRAF2 complexes were immunoprecipitated, and TRAF2 binding to endogenous cIAP1 and ectopically expressed cIAP2 was determined by Western blot. Asterisk indicates a nonspecific band. C, FLAG-TRAF2 CIM mutants showing reduced cIAP1/2 binding interact with RIPK1 and TRADD. 293T cells were transfected with FLAG-TRAF2 constructs and immunoprecipitated as in A, and binding to RIPK1 and TRADD was determined by Western blot. D, TRAF2-N domain binds directly to the cIAP1 BIR1 domain. The indicated glutathione _S_-transferase proteins were incubated with purified WT or mutant TRAF2-N domain protein as indicated, and following glutathione _S_-transferase (GST) immunoprecipitation, binding of the TRAF2-N domain (T2N) was detected by Coomassie staining.
FIGURE 3.
The TRAF2 CIM region 283–293 and RING domain are important for repression of constitutive noncanonical NF-κB activity caused by TRAF2 deletion. A, TRAF2 deletion results in constitutive NF-κB activity that is further enhanced by TNF stimulation. TRAF2 was deleted from parental TRAF2LoxP/LoxP conditional knock-out MEFs containing a lentiviral NF-κB GFP reporter by infection with a lentivirus harboring a Cre recombinase-expressing plasmid. TRAF2 parental WT and knock-out NF-κB GFP reporter cells were stimulated with TNF for 24 h, and reporter activity was measured by flow cytometry. Blue coloring, WT unstimulated cells; red coloring, TRAF2−/− unstimulated cells; green coloring, TNF-stimulated cells. B, WT but not CIM-mutated TRAF2 restores basal NF-κB GFP reporter activity in TRAF2−/− cells. TRAF2 expression in the TRAF2 conditional knock-out MEFs described in A was restored with the indicated WT and mutant constructs. Basal NF-κB reporter activity was measured by flow cytometry 72 h post-restoration of TRAF2 expression. Yellow coloring, parental WT MEFs; light blue coloring, TRAF2−/− MEFs; red coloring, uninduced TRAF2−/− cells reconstituted with the indicated inducible constructs; blue coloring, induced TRAF2−/− cells reconstituted with the indicated inducible constructs. The experiments shown are representative of results obtained on three independent occasions. C, TRAF2 deletion results in constitutive noncanonical NF-κB activity that is not reduced to normal levels by expression of TRAF2 CIM Δ283–293 or RING domain mutants. TRAF2−/− cells described in A were infected with the indicated inducible lentiviral TRAF2 constructs, and TRAF2 expression was induced in independent clones. 72 h post-induction, cells were harvested and analyzed by Western blot (WB) for the indicated proteins.
FIGURE 4.
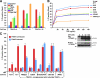
TRAF2 CIM residues 292EVE294 and 283–293 are required for TRAF2-mediated protection against TNF-induced death. A, immortalized TRAF2−/− cells are sensitized to TNF, but not FasL or TNF-related apoptosis-inducing ligand, -induced apoptosis. SV40 large T immortalized TRAF2 conditional knock-out MEFs were infected with Cre recombinase to delete TRAF2. Parental TRAF2LoxP/LoxP and TRAF2−/− MEFs were treated with TNF (60 ng/ml), FasL (10 ng/ml), TNF-related apoptosis-inducing ligand (TRAIL) (1 μg/ml), or compound A (500 n
m
) as indicated for 24 h, and cells death was quantified by PI staining and flow cytometry. Error bars are S.E. of at least three independent experiments. B, restoration of TRAF2 expression in TRAF2−/− cells restores TNF resistance. TRAF2−/− MEFs were infected with a lentiviral construct containing 4-hydroxytamoxifen-inducible WT murine TRAF2. TRAF2 expression was induced by the indicated doses of 4-hydroxytamoxifen (4HT) (Western blot (WB), bottom), and cell resistance to TNF (60 ng/ml) killing after 24 h was assessed by PI staining and flow cytometry. C, TRAF2 292EVE294 and Δ283–293 mutants do not protect against TNF killing in TRAF2−/− MEFs. TRAF2−/− cells were infected with the indicated 4-hydroxytamoxifen-inducible lentiviral TRAF2 constructs, and TRAF2 expression was induced for 48 h (see Fig. 3_C_ for TRAF2 expression levels). Cells were then treated with TNF (60 ng/ml) for a further 24 h, and cell death was compared with their uninduced counterparts by PI staining and flow cytometry. Error bars are S.E. of 3–5 independent experiments for several different clones.
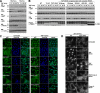
FIGURE 5.
TRAF2 CIM is important for TNF-induced RIPK1 ubiquitylation, but the TRAF2 RING domain is dispensable. The indicated MEFs were stimulated with FLAG-TNF (1 μg/ml) for 5 min, and the TNFR1 signaling complex precipitated with anti-FLAG antibody was described under “Experimental Procedures.” The eluted TNFR1 protein complex was examined by Western blot (WB) for the indicated proteins. To precipitate unstimulated TNFR1 (time = 0), FLAG-TNF was added to cell lysates. IP, immunoprecipitation.
FIGURE 6.
TRAF2 CIM is important for efficient TNF-induced NF-κB activation, but the TRAF2 RING domain is dispensable. A, TNF induction of NF-κB in TRAF2 knock-out MEFs. WT and TRAF2−/− cells were treated with TNF (60 ng/ml) for the indicated times, and cell lysates were analyzed by Western blot (WB). B, restoration of the normal NF-κB response to TNF in TRAF2/TRAF5 double knock-out MEFs requires the TRAF2 CIM but not the TRAF2 RING domain. The indicated cell types were treated with TNF (60 ng/ml) for the time periods shown, and cell lysates were analyzed by Western blot. C, TNF-induced translocation of p65 into the nucleus requires the TRAF2 CIM but not the TRAF2 RING domain. The indicated MEF cell lines were stimulated with TNF (60 ng/ml) for 20 min, and p65 localization was analyzed by immunostaining and fluorescence microscopy. DKO, TRAF2/TRAF5 double knock-out. D, delayed p65 nuclear translocation in TRAF2/TRAF5 double knock-out MEFs is prevented by IAP antagonist depletion of cIAP1/2. WT and TRAF2/TRAF5 double knock-out MEFs were treated with 60 ng/ml TNF for the indicated times with or without the IAP antagonist, compound A, and p65 localization was examined by immunofluorescence microscopy.
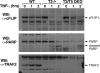
FIGURE 7.
Deletion of TRAF2 increases c-FLIPL loss upon TNF stimulation. The indicated MEF cell lines were stimulated with TNF for 0, 1, or 2 h, and cell lysates were examined by Western blot (WB) for the proteins shown.
Similar articles
- TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha.
Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, McKinlay M, Benetatos CA, Condon SM, Chunduru SK, Yeoh G, Brink R, Vaux DL, Silke J. Vince JE, et al. J Cell Biol. 2008 Jul 14;182(1):171-84. doi: 10.1083/jcb.200801010. Epub 2008 Jul 7. J Cell Biol. 2008. PMID: 18606850 Free PMC article. - Tumor necrosis factor (TNF) signaling, but not TWEAK (TNF-like weak inducer of apoptosis)-triggered cIAP1 (cellular inhibitor of apoptosis protein 1) degradation, requires cIAP1 RING dimerization and E2 binding.
Feltham R, Moulin M, Vince JE, Mace PD, Wong WW, Anderton H, Day CL, Vaux DL, Silke J. Feltham R, et al. J Biol Chem. 2010 Jun 4;285(23):17525-36. doi: 10.1074/jbc.M109.087635. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20356846 Free PMC article. - In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8.
Gentle IE, Wong WW, Evans JM, Bankovacki A, Cook WD, Khan NR, Nachbur U, Rickard J, Anderton H, Moulin M, Lluis JM, Moujalled DM, Silke J, Vaux DL. Gentle IE, et al. J Biol Chem. 2011 Apr 15;286(15):13282-91. doi: 10.1074/jbc.M110.216226. Epub 2011 Feb 21. J Biol Chem. 2011. PMID: 21339290 Free PMC article. - TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death.
Borghi A, Verstrepen L, Beyaert R. Borghi A, et al. Biochem Pharmacol. 2016 Sep 15;116:1-10. doi: 10.1016/j.bcp.2016.03.009. Epub 2016 Mar 16. Biochem Pharmacol. 2016. PMID: 26993379 Review. - Targeting signaling factors for degradation, an emerging mechanism for TRAF functions.
Yang XD, Sun SC. Yang XD, et al. Immunol Rev. 2015 Jul;266(1):56-71. doi: 10.1111/imr.12311. Immunol Rev. 2015. PMID: 26085207 Free PMC article. Review.
Cited by
- Programmed necrosis and autophagy in immune function.
Lu JV, Walsh CM. Lu JV, et al. Immunol Rev. 2012 Sep;249(1):205-17. doi: 10.1111/j.1600-065X.2012.01147.x. Immunol Rev. 2012. PMID: 22889224 Free PMC article. Review. - Oncogenic KEAP1 mutations activate TRAF2-NFκB signaling to prevent apoptosis in lung cancer cells.
Deen AJ, Adinolfi S, Härkönen J, Patinen T, Liu X, Laitinen T, Takabe P, Kainulainen K, Pasonen-Seppänen S, Gawriyski LM, Arasu UT, Selvarajan I, Mäkinen P, Laitinen H, Kansanen E, Kaikkonen MU, Poso A, Varjosalo M, Levonen AL. Deen AJ, et al. Redox Biol. 2024 Feb;69:103031. doi: 10.1016/j.redox.2024.103031. Epub 2024 Jan 4. Redox Biol. 2024. PMID: 38184997 Free PMC article. - Multitasking Kinase RIPK1 Regulates Cell Death and Inflammation.
Newton K. Newton K. Cold Spring Harb Perspect Biol. 2020 Mar 2;12(3):a036368. doi: 10.1101/cshperspect.a036368. Cold Spring Harb Perspect Biol. 2020. PMID: 31427374 Free PMC article. Review. - Expanding TRAF function: TRAF3 as a tri-faced immune regulator.
Häcker H, Tseng PH, Karin M. Häcker H, et al. Nat Rev Immunol. 2011 Jun 10;11(7):457-68. doi: 10.1038/nri2998. Nat Rev Immunol. 2011. PMID: 21660053 Review. - The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.
Moriwaki K, Chan FK. Moriwaki K, et al. Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
References
- Vaux D. L., Silke J. (2005) Nat. Rev. Mol. Cell Biol. 6, 287–297 - PubMed
- Imoto I., Yang Z. Q., Pimkhaokham A., Tsuda H., Shimada Y., Imamura M., Ohki M., Inazawa J. (2001) Cancer Res. 61, 6629–6634 - PubMed
- Tanimoto T., Tsuda H., Imazeki N., Ohno Y., Imoto I., Inazawa J., Matsubara O. (2005) Cancer Lett. 224, 141–151 - PubMed
- Dai Z., Zhu W. G., Morrison C. D., Brena R. M., Smiraglia D. J., Raval A., Wu Y. Z., Rush L. J., Ross P., Molina J. R., Otterson G. A., Plass C. (2003) Hum. Mol. Genet. 12, 791–801 - PubMed
- Imoto I., Tsuda H., Hirasawa A., Miura M., Sakamoto M., Hirohashi S., Inazawa J. (2002) Cancer Res. 62, 4860–4866 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous