Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson's disease - PubMed (original) (raw)
Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson's disease
Anna R Carta et al. J Neurochem. 2009 Dec.
Abstract
Adenosine A(2A) receptors antagonists produce neuroprotective effects in animal models of Parkinson's disease (PD). As neuroinflammation is involved in PD pathogenesis, both neuronal and glial A(2A) receptors might participate to neuroprotection. We employed complementary pharmacologic and genetic approaches to A(2A) receptor inactivation, in a multiple MPTP mouse model of PD, to investigate the cellular basis of neuroprotection by A(2A) antagonism. MPTP.HCl (20 mg/kg daily for 4 days) was administered in mice treated with the A(2A) antagonist SCH58261, or in conditional knockout mice lacking A(2A) receptors on forebrain neurons (fbnA(2A)KO mice). MPTP-induced partial loss of dopamine neurons in substantia nigra pars compacta (SNc) and striatum (Str), associated with increased astroglial and microglial immunoreactivity in these areas. Astroglia was similarly activated 1, 3, and 7 days after MPTP administration, whereas maximal microglial reactivity was detected on day 1, returning to baseline 7 days after MPTP administration. SCH58261 attenuated dopamine cell loss and gliosis in SNc and Str. Selective depletion of A(2A) receptors in fbnA(2A)KO mice completely prevented MPTP-induced dopamine neuron degeneration and gliosis in SNc, and partially counteracted gliosis in Str. Results provide evidence of a primary role played by neuronal A(2A) receptors in neuroprotective effects of A(2A) antagonists in a multiple MPTP injections model of PD. With the symptomatic antiparkinsonian potential of several A(2A) receptor antagonists being pursued in clinical trials, this study adds to the rationale for broader clinical benefit and use of these drugs early in the treatment of PD.
Figures
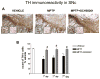
Fig 1. Adenosine A2A receptor antagonist SCH58261 prevents dopaminergic cell loss in the SNc
(A) shows representative sections immunostained for TH from SNc of mice sacrificed 3 days after MPTP treatment. Left insert shows TH-positive cells at higher magnification, right insert shows cresyl violet-stained sections; scale bar: 50 μm. Mice were treated with MPTP-HCl (20 mg/kg once a day for 4 days), plus SCH58261 (0.5 mg/kg) or vehicle (twice a day during MPTP treatment and once daily thereafter until sacrifice), and sacrificed 1, 3, 7 days after MPTP treatment. (B) shows analysis of TH immunostaining at 1, 3, 7 days after MPTP, reported as a percentage of TH-positive cells as compared to vehicle-treated mice. * indicates p<0.001 versus vehicle; # indicates p<0.001 versus MPTP group, by Tukey’s post hoc test. Scale bar: 50 μm.
Fig 2. Adenosine A2A receptor antagonist SCH58261 attenuates degeneration of dopaminergic terminals in the Str
(A) representative sections immunostained for TH, from the Str of mice sacrificed 3 days after MPTP treatment. (B) Results from pharmacological blockade with SCH58261 or genetic A2AR depletion are presented. In the left column, − and + indicate the administration of vehicle or SCH58261to MPTP-treated mice. In the right column + and − indicate fbnA2AWT and fbnA2AKO mice, respectively.
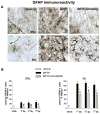
Fig 3. Adenosine A2A receptor antagonist SCH58261 counteracts astroglia activation in the SNc and Str
(A) shows representative sections immunostained for GFAP, from SNc (upper images) and Str (lower images) of mice sacrificed 3 days after MPTP treatment. Mice were treated as described in Fig 1. (B) shows analysis of GFAP immunostaining 1, 3, 7 days after MPTP, reported as percentage of GFAP-positive cells as compared to vehicle-treated mice in the SNc (left graph) and in the Str (right graph). * indicates p<0.001 versus corresponding vehicle and MPTP+SCH58261 groups; # indicates p<0.001 versus corresponding MPTP group, by Tukey’s post hoc test. Scale bar: 50 μm.
Fig 4. Adenosine A2A receptor antagonist SCH58261 counteracts microglia activation
(A) Representative images from the SNc immunostained for CD11b as a marker of microglia activation. Mice were treated as described in Fig 1 and sacrificed 1, 3, 7 days after MPTP treatment. (B) CD11b analysis in SNc and Str was performed in grey-scale digitized images. The area occupied by grey values above a threshold was calculated and expressed as square pixels and as percentage of vehicle-treated mice. Tukey’s post hoc test: * p<0.001 versus vehicle and MPTP+SCH58261 group; # p<0.001 versus MPTP group; ^, ^^ p<0.05, p<0.001 versus the indicated time point. Scale bar: 50 μm.
Fig 5. fbnA2A KO mice are protected against MPTP-induced loss of dopaminergic cells in the SNc
(A) shows representative sections from SNc immunostained for TH. Inserts show higher magnification of TH-labelled (left) and cresyl violet-labelled (right) cells. Mice were treated with MPTP (20 mg/kg once a day for 4 days)or vehicle. (B) shows analysis of TH immunostaining in fbnA2AKO mice, reported as a percentage of TH-positive cells as compared to vehicle-treated mice. Tukey’s post hoc test: * p<0.05 versus vehicle group; # p<0.05 versus WT MPTP group. Scale bar: 50 μm.
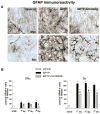
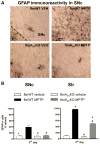
Fig 6. Astroglia activation is attenuated in SNc and Str of fbnA2AKO mice
(A) representative images from the SNc immunostained for GFAP, as a marker of astroglial cells. (B) Graphs show the analysis of GFAP immunostaining in SNc and Str, in fbnWT and fbnA2AKO mice treated with vehicle or MPTP. Tukey’s post hoc test: * p<0.001 versus vehicle group; # p<0.001 versus WT MPTP group. Scale bar: 50 μm.
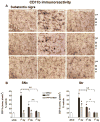
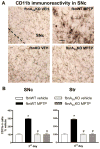
Fig 7. Microglia activation is prevented in SNc and Str of fbnA2AKO mice
(A) representative images from the SNc immunostained for CD11b, as a marker of microglia activation. (B) Graphs show the analysis of CD11b immunostaining in SNc and Str, in fbnWT and fbnA2AKO mice treated with vehicle or MPTP. Tukey’s post hoc test: * p<0.001 versus vehicle group; # p<0.001 versus WT MPTP group. Scale bar: 50 μm.
Similar articles
- Pathophysiological roles for purines: adenosine, caffeine and urate.
Morelli M, Carta AR, Kachroo A, Schwarzschild MA. Morelli M, et al. Prog Brain Res. 2010;183:183-208. doi: 10.1016/S0079-6123(10)83010-9. Prog Brain Res. 2010. PMID: 20696321 Free PMC article. Review. - Neuroprotective and anti-inflammatory effects of the adenosine A(2A) receptor antagonist ST1535 in a MPTP mouse model of Parkinson's disease.
Frau L, Borsini F, Wardas J, Khairnar AS, Schintu N, Morelli M. Frau L, et al. Synapse. 2011 Mar;65(3):181-8. doi: 10.1002/syn.20833. Synapse. 2011. PMID: 20665698 - MPTP-induced dopamine neuron degeneration and glia activation is potentiated in MDMA-pretreated mice.
Costa G, Frau L, Wardas J, Pinna A, Plumitallo A, Morelli M. Costa G, et al. Mov Disord. 2013 Dec;28(14):1957-65. doi: 10.1002/mds.25646. Epub 2013 Sep 20. Mov Disord. 2013. PMID: 24108425 - Systemically administered neuregulin-1β1 rescues nigral dopaminergic neurons via the ErbB4 receptor tyrosine kinase in MPTP mouse models of Parkinson's disease.
Depboylu C, Rösler TW, de Andrade A, Oertel WH, Höglinger GU. Depboylu C, et al. J Neurochem. 2015 May;133(4):590-7. doi: 10.1111/jnc.13026. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25581060 - Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease.
Watanabe Y, Himeda T, Araki T. Watanabe Y, et al. Med Sci Monit. 2005 Jan;11(1):RA17-23. Med Sci Monit. 2005. PMID: 15614202 Review.
Cited by
- Pathophysiological roles for purines: adenosine, caffeine and urate.
Morelli M, Carta AR, Kachroo A, Schwarzschild MA. Morelli M, et al. Prog Brain Res. 2010;183:183-208. doi: 10.1016/S0079-6123(10)83010-9. Prog Brain Res. 2010. PMID: 20696321 Free PMC article. Review. - Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration.
Kachroo A, Irizarry MC, Schwarzschild MA. Kachroo A, et al. Exp Neurol. 2010 Jun;223(2):657-61. doi: 10.1016/j.expneurol.2010.02.007. Epub 2010 Feb 24. Exp Neurol. 2010. PMID: 20188092 Free PMC article. - Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration.
Gordon R, Neal ML, Luo J, Langley MR, Harischandra DS, Panicker N, Charli A, Jin H, Anantharam V, Woodruff TM, Zhou QY, Kanthasamy AG, Kanthasamy A. Gordon R, et al. Nat Commun. 2016 Oct 5;7:12932. doi: 10.1038/ncomms12932. Nat Commun. 2016. PMID: 27703142 Free PMC article. - Deletion of adenosine A₁ or A(₂A) receptors reduces L-3,4-dihydroxyphenylalanine-induced dyskinesia in a model of Parkinson's disease.
Xiao D, Cassin JJ, Healy B, Burdett TC, Chen JF, Fredholm BB, Schwarzschild MA. Xiao D, et al. Brain Res. 2011 Jan 7;1367:310-8. doi: 10.1016/j.brainres.2010.08.099. Epub 2010 Sep 7. Brain Res. 2011. PMID: 20828543 Free PMC article. - The Pharmacological Potential of Adenosine A2A Receptor Antagonists for Treating Parkinson's Disease.
Mori A, Chen JF, Uchida S, Durlach C, King SM, Jenner P. Mori A, et al. Molecules. 2022 Apr 6;27(7):2366. doi: 10.3390/molecules27072366. Molecules. 2022. PMID: 35408767 Free PMC article. Review.
References
- Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033:216–20. - PubMed
- Barcia C, Sánchez Bahillo A, Fernández-Villalba E, Bautista V, Poza Y, Poza M, Fernández-Barreiro A, Hirsch EC, Herrero MT. Glia. Vol. 46. 2004. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure; pp. 402–09. - PubMed
- Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology. 2005;30:891–900. - PubMed
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–35. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous