Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion - PubMed (original) (raw)
. 2010 Jan 15;85(2):385-94.
doi: 10.1093/cvr/cvp334. Epub 2009 Oct 10.
Affiliations
- PMID: 19820255
- PMCID: PMC2797452
- DOI: 10.1093/cvr/cvp334
Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion
Eric N Churchill et al. Cardiovasc Res. 2010.
Abstract
Aims: The response of the myocardium to an ischaemic insult is regulated by two highly homologous protein kinase C (PKC) isozymes, delta and epsilonPKC. Here, we determined the spatial and temporal relationships between these two isozymes in the context of ischaemia/reperfusion (I/R) and ischaemic preconditioning (IPC) to better understand their roles in cardioprotection.
Methods and results: Using an ex vivo rat model of myocardial infarction, we found that short bouts of ischaemia and reperfusion prior to the prolonged ischaemic event (IPC) diminished deltaPKC translocation by 3.8-fold and increased epsilonPKC accumulation at mitochondria by 16-fold during reperfusion. In addition, total cellular levels of deltaPKC decreased by 60 +/- 2.7% in response to IPC, whereas the levels of epsilonPKC did not significantly change. Prolonged ischaemia induced a 48 +/- 11% decline in the ATP-dependent proteasomal activity and increased the accumulation of misfolded proteins during reperfusion by 192 +/- 32%; both of these events were completely prevented by IPC. Pharmacological inhibition of the proteasome or selective inhibition of epsilonPKC during IPC restored deltaPKC levels at the mitochondria while decreasing epsilonPKC levels, resulting in a loss of IPC-induced protection from I/R. Importantly, increased myocardial injury was the result, in part, of restoring a deltaPKC-mediated I/R pro-apoptotic phenotype by decreasing pro-survival signalling and increasing cytochrome c release into the cytosol.
Conclusion: Taken together, our findings indicate that IPC prevents I/R injury at reperfusion by protecting ATP-dependent 26S proteasomal function. This decreases the accumulation of the pro-apoptotic kinase, deltaPKC, at cardiac mitochondria, resulting in the accumulation of the pro-survival kinase, epsilonPKC.
Figures
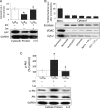
Figure 1
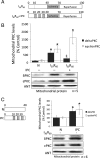
Ischaemic preconditioning decreases δPKC and increases εPKC levels at cardiac mitochondria during reperfusion. (A) Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Hearts were then removed, homogenized, fractionated, and the mitochondrial fraction was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to adenine nucleotide translocase (ANT), a mitochondrial marker, and expressed as % of normoxia (N) control. (B) Western blot analysis of mitochondrial protein showing that 30 min of ischaemia followed by 60 min of reperfusion (I30R60) resulted in translocation of δPKC to mitochondria (six-folds increase; P < 0.05 vs. N), which was blocked when hearts were preconditioned (IPC) before the global ischaemic event (P < 0.05 vs. I30R60). Likewise, εPKC association with cardiac mitochondria increased ∼7-folds during I30R60 (P < 0.05 vs. N), but in contrast to δPKC, IPC prior to I/R further increased translocation of εPKC to 16-folds over the levels seen in normoxic hearts (P < 0.05 vs. N and I30R60). (C) Western blot analyses showing that the IPC stimulus alone (without I30R60) significantly increased translocation of δPKC (P < 0.05 vs. N) and εPKC translocation (P < 0.05 vs. N) to the mitochondria. *P < 0.05 vs. N, ‡P < 0.05 vs. I30R60) Translocation of δPKC and εPKC to the mitochondria were analysed by one-way analysis of variance with a post-hoc Tukey test. Mitochondrial PKC levels were analysed by Student's _t_-test.
Figure 2
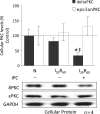
Diminished mitochondrial levels of δPKC following IPC are due to decreased cellular levels of the isozyme. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols in Figure 1. Hearts were then removed, and homogenized, and the total homogenate was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to glyceraldehyde phosphate dehydrogenase (GAPDH), a cytosolic protein, and expressed as %N. I30R60 had no significant effect on total levels of either δPKC or εPKC. However, IPC before the global ischaemic event decreased the overall levels of δPKC by ∼80% (P < 0.05 vs. N), but not εPKC levels. *P < 0.05 vs. Normoxia and ‡P < 0.05 vs. I30R60. Cellular δPKC and εPKC levels were analysed by one-way analysis of variance with a post-hoc Tukey test.
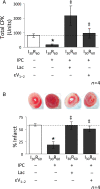
Figure 3
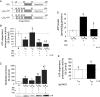
Effect of preconditioning on ischaemia-induced loss in ATP-dependent proteasome activity. (A) Cytosolic extracts were prepared from hearts exposed to 70 min of normoxic perfusion (N), 30 min of ischaemia (I30), or three cycles of preconditioning (5 min ischaemia and 5 min reperfusion) followed by 30 min of ischaemia (I30 + IPC) in the absence or presence of the proteasome inhibitor lactacystin or the specific εPKC inhibitor εV1–2. Chymotrypsin-like activity of the proteasome present in the cytoplasmic milieu was evaluated and the specific inhibitor MG-132 (20 μM) was utilized to ensure that measured activities were due to the proteasome (data not shown). The presence of unfolded proteins was evaluated using the slot blot technique with an anti-soluble oligomer antibody. Values representing ATP-dependent proteasome activity and misfolded proteins are presented as a percent of values obtained with samples from hearts exposed to 60 min of normoxic perfusion (N). Values represent the mean ± standard deviation (n = 4). (B) Ischaemia resulted in a 50% decline in ATP-dependent proteasome activity (P < 0.05 vs. N), which was completely reversed by IPC (P < 0.05 vs. I30). The proteasome inhibitor, lactacystin (2 μM) and the specific εPKC inhibitor (1μM εV1–2) both significantly decreased the activity of the proteasome (P < 0.05 vs. I30R60 + IPC; n = 4). (C) I30R60 resulted in an ∼3-fold increase in misfolded proteins which was prevented by IPC (P < 0.05; n = 4). Treatment of hearts with lactacystin or εV1–2 blocked the effect of IPC and increased the accumulation of misfolded proteins. (D) IPC elevated ATP levels by 3.5-fold in hearts that had undergone I30R60, and εV1–2 blocked these effects. (E) Treatment with the εPKC activator (ψεRACK) protected the proteasome from ischaemia-mediated inhibition (P < 0.05 vs. I30). *P < 0.05 vs. Normoxia, +P = 0.05 vs. I30R60, §P < 0.05 vs. I30, ‡P < 0.05 vs. IPC + I30, †P < 0.05 vs. IPC + I30R60; Misfolded protein accumulation and proteasome activity were analysed by one-way analysis of variance with a post-hoc Tukey test. Figure 3D, proteasome activity was analysed by Student's _t_-test.
Figure 4
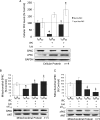
Inhibition of the proteasome restores δPKC cellular and mitochondrial levels in IPC hearts with a resultant decrease in εPKC levels. (A) Hearts were hung in Langendorff mode and treated with the above-mentioned perfusion protocols. Hearts were then removed, homogenized, and the total homogenate and mitochondrial fractions were subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to GAPDH (total homogenate) or ANT (mitochondrial fraction) and expressed as % I30R60. IPC before prolonged ischaemia reduced total levels of δPKC by ∼80% (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin blocked δPKC degradation (P < 0.05 vs. I30R60 + IPC). Similar to Figure 2, IPC before prolonged ischaemia did not significantly change overall levels of εPKC. However, inhibition of the proteasome increased εPKC levels by ∼2-folds (P < 0.05 vs. I30R60). Inhibition of εPKC activity with εV1–2 did not significantly change the overall levels of either δ or εPKC isozymes (data not shown). (B) As in Figure 2, IPC before I30R60 decreased levels of δPKC at mitochondria by ∼60% (P < 0.05 vs. I30R60). This was completely prevented in hearts treated with 2 μM lactacystin and 1 μM εV1–2 (P < 0.05 vs. I30R60 + IPC). (C) Although δPKC mitochondrial levels were restored, εPKC levels were diminished by 40% relative to I30R60 and by 60% relative to I30R60 + IPC (P < 0.05). Hearts that were treated with 1 μM of a peptide inhibitor of εPKC (εV1–2) during the IPC protocol showed a significant decrease in εPKC mitochondrial levels (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Cellular and mitochondrial PKC levels were analysed by the one-way analysis of variance with a post-hoc testing by Tukey.
Figure 5
Inhibition of δPKC degradation restores the apoptotic phenotype seen during reperfusion. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Hearts were then removed, homogenized, fractionated, and the cytosolic homogenate was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to GAPDH and expressed as % I30R60. (A) IPC before prolonged ischaemia significantly decreased cytochrome c release into the cytosol (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin restored cytochrome c release to levels seen during I30R60 (P < 0.05 vs. I30R60 + IPC). (B) Ischaemia alone and perfusion with lactacystin or εV1–2 alone did not result in significant release of cytochrome c into the cytosol. Additionally, as evidenced by a lack of mitochondrial VDAC in the cytosolic fraction, there was little contamination from mitochondrial cytochrome c in this fraction. Enolase was used as a cytosolic loading control. (C) IPC before prolonged ischaemia also increased phosphorylation of the pro-survival kinase, Akt, by ∼3-fold (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin decreased phosphorylation back to I30R60 levels (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Cytosolic cytochrome c levels and p-Akt were analysed by one-way analysis of variance with a post-hoc Tukey test.
Figure 6
Inhibition of the proteasome reverses the IPC-mediated protective effects on tissue injury. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Tissue injury was determined by measuring the release of CPK into the cardiac effluent (total CPK units). Following removal, hearts were sliced and stained with TTC to differentiate between necrotic (stained white) and viable (stained red) tissue (% infarct). (A,B) Hearts subjected to I30R60 showed an increase in both CPK release and myocardial infarction and both were blocked by IPC (reductions of ∼60% for CPK release and ∼40% for infarction, respectively). Perfusion of 2 μM lactacystin during the IPC protocol and for the first 10 min of reperfusion reversed this effect resulting in significantly higher levels of CPK release (P < 0.05 vs. I30R60 + IPC) and myocardial infarction (P < 0.05 vs. I30R60 + IPC). Similar to proteasome inhibition, εPKC inhibition (1 μM εV1–2) also significantly increased both CPK release (P < 0.05 vs. I30R60 + IPC) and myocardial infarction (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Total CPK and % infarcted area were analysed by the one-way analysis of variance with a post-hoc Tukey test.
Similar articles
- Angiotensin II-preconditioning is associated with increased PKCε/PKCδ ratio and prosurvival kinases in mitochondria.
Nuñez RE, Javadov S, Escobales N. Nuñez RE, et al. Clin Exp Pharmacol Physiol. 2017 Dec;44(12):1201-1212. doi: 10.1111/1440-1681.12816. Epub 2017 Sep 20. Clin Exp Pharmacol Physiol. 2017. PMID: 28707739 Free PMC article. - Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury.
Budas GR, Churchill EN, Mochly-Rosen D. Budas GR, et al. Pharmacol Res. 2007 Jun;55(6):523-36. doi: 10.1016/j.phrs.2007.04.005. Epub 2007 Apr 29. Pharmacol Res. 2007. PMID: 17576073 Review. - The significance of the washout period in preconditioning.
Salie R, Lochner A, Loubser DJ. Salie R, et al. Cardiovasc Ther. 2017 Jun;35(3). doi: 10.1111/1755-5922.12252. Cardiovasc Ther. 2017. PMID: 28118517 - Cyclosporine-A mimicked the ischemic pre- and postconditioning-mediated cardioprotection in hypertensive rats: Role of PKCε.
González Arbeláez LF, Ciocci Pardo A, Fantinelli JC, Mosca SM. González Arbeláez LF, et al. Exp Mol Pathol. 2016 Apr;100(2):266-75. doi: 10.1016/j.yexmp.2016.01.009. Epub 2016 Feb 1. Exp Mol Pathol. 2016. PMID: 26844384 - The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury.
Churchill EN, Mochly-Rosen D. Churchill EN, et al. Biochem Soc Trans. 2007 Nov;35(Pt 5):1040-2. doi: 10.1042/BST0351040. Biochem Soc Trans. 2007. PMID: 17956273 Review.
Cited by
- Fenofibrate attenuates impaired ischemic preconditioning-mediated cardioprotection in the fructose-fed hypertriglyceridemic rat heart.
Babbar L, Mahadevan N, Balakumar P. Babbar L, et al. Naunyn Schmiedebergs Arch Pharmacol. 2013 Apr;386(4):319-29. doi: 10.1007/s00210-012-0830-3. Epub 2013 Jan 17. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23325365 - An Update on Protein Kinases as Therapeutic Targets-Part I: Protein Kinase C Activation and Its Role in Cancer and Cardiovascular Diseases.
Silnitsky S, Rubin SJS, Zerihun M, Qvit N. Silnitsky S, et al. Int J Mol Sci. 2023 Dec 18;24(24):17600. doi: 10.3390/ijms242417600. Int J Mol Sci. 2023. PMID: 38139428 Free PMC article. Review. - Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins.
Ranek MJ, Terpstra EJ, Li J, Kass DA, Wang X. Ranek MJ, et al. Circulation. 2013 Jul 23;128(4):365-76. doi: 10.1161/CIRCULATIONAHA.113.001971. Epub 2013 Jun 14. Circulation. 2013. PMID: 23770744 Free PMC article. - Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training.
Campos JC, Fernandes T, Bechara LR, da Paixão NA, Brum PC, de Oliveira EM, Ferreira JC. Campos JC, et al. Oxid Med Cell Longev. 2015;2015:464195. doi: 10.1155/2015/464195. Epub 2015 Apr 14. Oxid Med Cell Longev. 2015. PMID: 25954323 Free PMC article. - Post-translational modification of cardiac proteasomes: functional delineation enabled by proteomics.
Scruggs SB, Zong NC, Wang D, Stefani E, Ping P. Scruggs SB, et al. Am J Physiol Heart Circ Physiol. 2012 Jul;303(1):H9-18. doi: 10.1152/ajpheart.00189.2012. Epub 2012 Apr 20. Am J Physiol Heart Circ Physiol. 2012. PMID: 22523251 Free PMC article. Review.
References
- Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. - PubMed
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. - PubMed
- Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. - PubMed
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. - PubMed