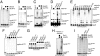Identification of a physiological E2 module for the human anaphase-promoting complex - PubMed (original) (raw)
Identification of a physiological E2 module for the human anaphase-promoting complex
Adam Williamson et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Ubiquitination by the anaphase-promoting complex (APC/C) is essential for proliferation in all eukaryotes. The human APC/C promotes the degradation of mitotic regulators by assembling K11-linked ubiquitin chains, the formation of which is initiated by its E2 UbcH10. Here, we identify the conserved Ube2S as a K11-specific chain elongating E2 for human and Drosophila APC/C. Ube2S depends on the cell cycle-dependent association with the APC/C activators Cdc20 and Cdh1 for its activity. While depletion of Ube2S already inhibits APC/C in cells, the loss of the complete UbcH10/Ube2S-module leads to dramatic stabilization of APC/C substrates, severe spindle defects, and a strong mitotic delay. Ube2S and UbcH10 are tightly co-regulated in the cell cycle by APC/C-dependent degradation. We conclude that UbcH10 and Ube2S constitute a physiological E2-module for APC/C, the activity of which is required for spindle assembly and cell division.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Ube2S catalyzes the formation of K11-linked ubiquitin chains on APC/C-substrates. (A) Ube2S promotes chain formation on cyclin A. 35S-cyclin A was incubated with APC/CCdh1, E1, ubiquitin, and UbcH10, and/or Ube2S. Reaction products were analyzed by autoradiography. (B) Ube2S promotes chain formation on Plk1. The ubiquitination of 35S-Plk1 was analyzed as described for cyclin A. (C) Ube2S does not promote chain initiation. 35S-cyclin A and 35S-Plk1 were incubated with APC/CCdh1 and methyl-ubiquitin, as described above. (D) Ube2S cooperates with APC/CCdc20. APC/CCdc20 was activated as described (23), and used to ubiquitinate endogenous Cdc20, as detected by Western blot. (E) Ube2S assembles K11-linked chains on APC/C substrates. The ubiquitination of 35S-cyclin A by APC/CCdh1 and Ube2S was analyzed in the presence of ubiquitin mutants, as indicated. “K6” describes ubiquitin containing Lys-6, “R6” is ubiquitin, in which K6 has been exchanged to Arg. (F) Ube2S requires its catalytic Cys and its C terminus for APC/C-dependent chain formation. Ubiquitination of 35S-cyclin A was analyzed in the presence of APC/CCdh1, Ube2S, Ube2SC95S, or Ube2S1–196/Ube2SΔC. In addition, the activity of Ube2S was competed by a C-terminal peptide encompassing the last 26 amino acids of Ube2S (Ube2S+pep). (G) Ube2S catalyzes formation of K11-linked ubiquitin dimers independently of an E3. Ube2S and indicated mutants were incubated with E1 and ubiquitin or ubi-R11, and the formation of ubiquitin dimers (ubi-ubi) was detected by Coomassie staining. (H) Ube2S does not require UbcH10 during chain elongation. 35S-cyclin A was briefly incubated with APC/CCdh1 and UbcH10, before UbcH10 was inactivated by adding a 100-fold excess of UbcH10C114S. Then, Ube2S was added alone or together with the APC/C inhibitor Emi1. (I) Ube2S depends on the APC/C for activity. 35S-cyclin A was briefly incubated with APC/CCdh1 and UbcH10, and then Ube2S alone or Ube2S together with the APC/C inhibitor Emi1 or an excess of the APC/C substrate securin were added.
Fig. 2.
Ube2S interacts with APC/C. (A) Ube2S binds Cdc20 and Cdh1 in a cell cycle-dependent manner. Ube2S was precipitated from extracts of synchronized HeLa cells, and co-purifying proteins were detected by Western blot. (B) Cdc20 binds Ube2S in mitosis. Cdc20 was precipitated from mitotic HeLa S3 cells. Co-purifying Ube2S and Mad2 were detected by Western blot. (C) Ube2S binds Cdh1. FLAGUbe2S was precipitated from 293T cells, and co-purifying HACdh1 was detected by Western blot. (D) Cdh1 binds Ube2S. HACdh1 or HACdh1ΔWD40 were precipitated from 293T cells, and co-purifying Ube2S was detected by Western blot. (E) Ube2S binds Cdh1 from G1 extracts. Immobilized MBP or MBPUbe2S were incubated with HeLa S3 extracts. Recombinant securin or UbcH10 (≈100× excess over Cdh1) were added as indicated. Bound Cdh1 was detected by Western blot. (F) Ube2S binds Cdh1. 35S-Cdh1 was incubated with immobilized MBPUbe2S or the indicated truncation mutants. Bound Cdh1 was detected by autoradiography. (G) Ube2S directly binds Cdh1. HisCdh1 purified to homogeneity was incubated with MBP and MBPUbe2S. Bound Cdh1 was detected by Western blot. (H) The C terminus of Ube2S is required for its interaction with Cdh1. FLAGUbe2S or FLAGUbe2SΔC were precipitated from 293T cells, and bound HACdh1 was analyzed by Western blot.
Fig. 3.
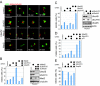
Ube2S regulates progression of cells through mitosis. (A) Drosophila Ube2S is required for mitosis. S2 cells stably expressing histone H2B-GFP and α-tubulin-mCherry were transfected with RNAi and filmed by time-lapse microscopy. (B) Ube2S and UbcH10 cooperate to promote progression through mitosis. HeLa cells were transfected with the indicated siRNAs, and scored for mitotic cells. Error bars, SEM derived from at least seven independent frames of different experiments, counting at least 1,000 cells in each frame. The right panel shows the depletion efficiency of Western blot. (C) UbcH10 shows genetic interactions with Ube2S, but not UbcH5. UbcH10 was depleted by siRNA alone, together with all four UbcH5-homologs, or with Ube2S, and the mitotic index of the cell population was determined. The right panel shows the depletion efficiency, as detected by Western blot. (D) Ube2S contributes to APC/C-dependent spindle checkpoint silencing. HeLa cells were transfected with the indicated siRNA and scored for cells arresting in mitosis. (E) Ube2S counteracts the DUB Usp44. HeLa cells were transfected with indicated siRNA, and treated with taxol to activate the spindle checkpoint. The number of cells arrested in mitosis were counted.
Fig. 4.
Ube2S and UbcH10 promote the degradation of APC/C substrates in cells. (A) Depletion of Ube2S and UbcH10 strongly stabilizes APC/C substrates. Mitotic HeLa cells transfected with siRNA against UbcH10 and Ube2S were released into G1, and the levels of several APC/C-substrates were measured by Western blot. (B) The UbcH10/Ube2S module drives degradation of cyclin A. HeLa cells were transfected with control or UbcH10/Ube2S siRNA, and cyclin A was detected by immunofluorescence (green). The spindle was visualized by antibodies against α-tubulin (red), and DNA was stained with DAPI (blue). (Scale bar, 10 μm.) The right panel shows the quantification of mitotic HeLa cells before anaphase with detectable cyclin A staining on the spindle. (C) Accumulation of Tpx2 in postmitotic HeLa cells after depletion of Ube2S and UbcH10. Tpx2 was detected in HeLa cells transfected with the indicated siRNAs by immunofluorescence. Cells in early G1 were identified, and representative images are shown. (Scale bar, 10 μm.) The right panel shows a quantification of Tpx2 levels in HeLa cells in G1. (D) Ube2S is required for degradation of cyclin B in Drosophila. S2 cells stably expressing cyclin B-GFP were transfected with the RNAi, and imaged by time-lapse microsopy. Depletion of Ube2S stabilizes cyclin B-GFP on the spindle pole (arrow) independently of spindle checkpoint activation.
Fig. 5.
Ube2S is regulated by the APC/C. (A) Ube2S is degraded during G1. Mitotic HeLa cells were released into fresh medium to allow mitotic exit, and the indicated proteins were detected by Western blot. (B) Ube2S levels decrease upon activation of the APC/C. HeLa cells were treated with siRNA against the APC/C inhibitor Emi1, and the indicated proteins were detected by Western blot. (C) Ube2S-levels increase after depletion of APC/C subunits. HeLa cells were treated with the indicated siRNAs, and the levels of Ube2S were determined by Western blotting. (D) Ube2S is ubiquitinated by APC/CCdh1. Affinity-purified APC/CCdh1 was incubated with 35S-Ube2S in the absence or presence of UbcH5c, UbcH10, or Emi1. Reaction products were detected by autoradiography. (E) Microtubules promote the ubiquitination of Ube2S. APC/CCdh1 was incubated with taxol-stabilized microtubules, UbcH5c, and 35S-Ube2S, 35S-Ube2SC95S, 35S-Ube2SΔC, or 35S-Ube2S, and the C-terminal Ube2S-peptide. Reaction products were detected by autoradiography. (F) Ubiquitinated Ube2S is degraded by the 26S proteasome. 35S-Ube2S was ubiquitinated by APC/CCdh1 in the presence of microtubules. Subsequent to the ubiquitination, purified 26S proteasomes were added. MG132 was added as indicated. The reaction products were visualized by autoradiography.
Similar articles
- UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex.
Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW. Wu T, et al. Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1355-60. doi: 10.1073/pnas.0912802107. Epub 2010 Jan 6. Proc Natl Acad Sci U S A. 2010. PMID: 20080579 Free PMC article. - UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit.
Garnett MJ, Mansfeld J, Godwin C, Matsusaka T, Wu J, Russell P, Pines J, Venkitaraman AR. Garnett MJ, et al. Nat Cell Biol. 2009 Nov;11(11):1363-9. doi: 10.1038/ncb1983. Epub 2009 Oct 11. Nat Cell Biol. 2009. PMID: 19820702 Free PMC article. - Efficient APC/C substrate degradation in cells undergoing mitotic exit depends on K11 ubiquitin linkages.
Min M, Mevissen TE, De Luca M, Komander D, Lindon C. Min M, et al. Mol Biol Cell. 2015 Dec 1;26(24):4325-32. doi: 10.1091/mbc.E15-02-0102. Epub 2015 Oct 7. Mol Biol Cell. 2015. PMID: 26446837 Free PMC article. - Impressionist portraits of mitotic exit: APC/C, K11-linked ubiquitin chains and Cezanne.
Bonacci T, Emanuele MJ. Bonacci T, et al. Cell Cycle. 2019 Mar-Apr;18(6-7):652-660. doi: 10.1080/15384101.2019.1593646. Epub 2019 Mar 28. Cell Cycle. 2019. PMID: 30874463 Free PMC article. Review. - Processive ubiquitin chain formation by the anaphase-promoting complex.
Meyer HJ, Rape M. Meyer HJ, et al. Semin Cell Dev Biol. 2011 Aug;22(6):544-50. doi: 10.1016/j.semcdb.2011.03.009. Epub 2011 Apr 6. Semin Cell Dev Biol. 2011. PMID: 21477659 Free PMC article. Review.
Cited by
- RING E3 mechanism for ubiquitin ligation to a disordered substrate visualized for human anaphase-promoting complex.
Brown NG, VanderLinden R, Watson ER, Qiao R, Grace CR, Yamaguchi M, Weissmann F, Frye JJ, Dube P, Ei Cho S, Actis ML, Rodrigues P, Fujii N, Peters JM, Stark H, Schulman BA. Brown NG, et al. Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5272-9. doi: 10.1073/pnas.1504161112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825779 Free PMC article. - Regulatory mechanisms of kinetochore-microtubule interaction in mitosis.
Tanaka K. Tanaka K. Cell Mol Life Sci. 2013 Feb;70(4):559-79. doi: 10.1007/s00018-012-1057-7. Epub 2012 Jul 4. Cell Mol Life Sci. 2013. PMID: 22752158 Free PMC article. Review. - UBE2S is associated with malignant characteristics of breast cancer cells.
Ayesha AK, Hyodo T, Asano E, Sato N, Mansour MA, Ito S, Hamaguchi M, Senga T. Ayesha AK, et al. Tumour Biol. 2016 Jan;37(1):763-72. doi: 10.1007/s13277-015-3863-7. Epub 2015 Aug 6. Tumour Biol. 2016. PMID: 26245992 - Taming the Beast: Control of APC/CCdc20-Dependent Destruction.
Lara-Gonzalez P, Kim T, Desai A. Lara-Gonzalez P, et al. Cold Spring Harb Symp Quant Biol. 2017;82:111-121. doi: 10.1101/sqb.2017.82.033712. Epub 2017 Nov 13. Cold Spring Harb Symp Quant Biol. 2017. PMID: 29133301 Free PMC article. - The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation.
Jia L, Li B, Yu H. Jia L, et al. Nat Commun. 2016 Feb 25;7:10818. doi: 10.1038/ncomms10818. Nat Commun. 2016. PMID: 26912231 Free PMC article.
References
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. - PubMed
- Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–150. - PubMed
- Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. - PubMed
- Eddins MJ, Carlile CM, Gomez KM, Pickart CM, Wolberger C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat Struct Mol Biol. 2006;13:915–920. - PubMed
- Peters JM. The anaphase-promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous