Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition - PubMed (original) (raw)
Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition
Paramita Chakrabarty et al. FASEB J. 2010 Feb.
Abstract
Proinflammatory stimuli, after amyloid beta (Abeta) deposition, have been hypothesized to create a self-reinforcing positive feedback loop that increases amyloidogenic processing of the Abeta precursor protein (APP), promoting further Abeta accumulation and neuroinflammation in Alzheimer's disease (AD). Interleukin-6 (IL-6), a proinflammatory cytokine, has been shown to be increased in AD patients implying a pathological interaction. To assess the effects of IL-6 on Abeta deposition and APP processing in vivo, we overexpressed murine IL-6 (mIL-6) in the brains of APP transgenic TgCRND8 and TG2576 mice. mIL-6 expression resulted in extensive gliosis and concurrently attenuated Abeta deposition in TgCRND8 mouse brains. This was accompanied by up-regulation of glial phagocytic markers in vivo and resulted in enhanced microglia-mediated phagocytosis of Abeta aggregates in vitro. Further, mIL-6-induced neuroinflammation had no effect on APP processing in TgCRND8 and had no effect on APP processing or steady-state levels of Abeta in young Tg2576 mice. These results indicate that mIL-6-mediated reactive gliosis may be beneficial early in the disease process by potentially enhancing Abeta plaque clearance rather than mediating a neurotoxic feedback loop that exacerbates amyloid pathology. This is the first study that methodically dissects the contribution of mIL-6 with regard to its potential role in modulating Abeta deposition in vivo.
Figures
Figure 1.
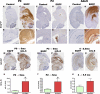
AAV1-mediated transduction of mIL-6 in mouse brain results in widespread expression and robust gliosis. A–L) AAVI-EGFP (2×109 genome particles/ventricle) was injected into the cerebral ventricles of TgCRND8 pups on day P0 (A_–_D, P0) or P2 (E_–_H, P2) or injected stereotaxically into the hippocampus of 4-mo-old mice (I_–_L, Adult). Mice were then sacrificed after 4–6 wk (_n_=5/group). Age-matched controls were injected with saline in all cases. Representative images of whole-brain sections (top panels) and hippocampus (bottom panels) show widespread EGFP expression in both forebrain and hindbrain areas in P0-injected mice (A_–_D), whereas P2 injection results in localized transduction of the choroid plexus (E_–_H). AAVI-EGFP injection into the hippocampus of 4-mo-old mice results in transduction of the hippocampal pyramidal layer as well as neuronal projections in the cortex and thalamus (I_–_L) at least 1 mm anterior and posterior to the point of injection. M–R) AAV1-mIL-6 or AAV1-EGFP (2×109 genome particles/ventricle) was injected into the cerebral ventricles of TgCRND8 mice on either P0 (M, N; P0→5 mo) or P2 (O, P; P2→5 mo) and sacrificed after 5 mo (_n_=9–12/group). GFAP immunostaining shows increased astrogliosis in both P0 → 5 mo and P2 → 5 mo mIL-6-injected mice compared with EGFP-injected control mice. Stereotactic injection of AAV1 mIL-6 into the hippocampus of 4-mo TgCRND8 mice and analyzed after 6 wk (Q, R; 4→5.5 mo) shows increased astrogliosis (_n_=5–6/group). S–U) Levels of mIL-6 in injected mouse brains were analyzed using sandwich ELISA technique using RIPA-soluble brain lysates. Results are expressed as fold over control (_n_=5/group). *P < 0.05. Scale bars = 600 μm (A, C, E, G, M–P); 500 μm (I, K, Q, R); 150 μm (B, D); 250 μm (F, H); 125 μm (J, L).
Figure 2.
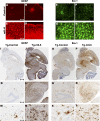
AAV1-mIL-6 expression in transgenic CRND8 mice results in extensive induction of astrogliosis and microgliosis. A–H) Up-regulation of activated astrocytes (C, D; GFAP) and microglia (G, H; Iba-1) in the cortex of P0 → mIL-6-injected TgCRND8 mice compared with control TgCRND8 mice (A, B; GFAP; E, F; Iba-1) detected by immunofluorescent staining of free-floating fixed sections (GFAP, red; Iba-1, green). I–N) Reactive astrocytes (GFAP immunoreactivity) in paraffin-embedded sections of P0 → 5 mo TgCRND8 mice injected with either mIL-6 (Tg-mIL6) or EGFP (Tg-Control). Whole-brain sections (I, J) along with higher magnification pictures (K_–_N, bottom panels) show detailed morphology of the activated astrocytes in and around the corresponding hippocampus. O–T) Iba-1 immunoreactivity in whole brain sections (O_–_P) and higher magnifications of the hippocampus (Q_–_T, bottom panels) in P0 → 5 mo TgCRND8 mice. Abundant activated microglia displaying hypertrophic processes are present in mIL-6-injected mice (Tg-mIL6) compared with EGFP-expressing control mice (Tg-Control). Scale bars = 50 μm (A–H); 600 μm (I, J, O, P), 150 μm (K, L, Q, R), and 25 μm (M, N, S, T).
Figure 3.
Attenuation of Aβ deposition in AAV1-mIL-6-expressing TgCRND8 mice. A–F). Representative brain sections stained with pan-Aβ1–16 antibody (mAb 33.1.1) show Aβ plaque immunoreactivity in the hippocampus of P0 → 5 mo mIL-6-expressing (C, D; P0-mIL-6), P2 → 5 mo mIL-6-expressing (E, F; P2-mIL-6), and age-matched P0 → 5 mo EGFP-expressing TgCRND8 mice (A, B; Control). G) There was a significant decrease in total forebrain Aβ as well as hippocampal Aβ plaque burdens in both P0 → 5 mo and P2 → 5 mo injection groups compared with control mice. H–I) Biochemical analyses of FA extractable Aβ42 and Aβ40 levels in P0 → 5 mo mIL-6-expressing TgCRND8 mice (H) and P2 → 5 mo mIL-6-expressing CRND8 mice (I) compared with EGFP-expressing age matched controls. J–M) 4-mo-old TgCRND8 mice were stereotaxically injected in the hippocampus with either AAV1-mIL-6 or AAV1-EGFP and sacrificed after 6 wk (_n_=5–6/group). Representative brain sections stained with 33.1.1 antibody (pan-Aβ 1–16) depict attenuation of Aβ deposition in mIL-6-expressing mice (L, M; Adult mIL-6) compared with controls (J, K; Control) in the immediate vicinity of the injection site. N) Aβ plaque burden analysis shows a significant decrease in amyloid deposition in mIL-6-injected mice compared with control EGFP-injected mice O) Biochemical analyses of Aβ42 and Aβ40 levels by ELISA show significant reductions in FA fraction in mIL-6-injected mice compared with controls. *P < 0.05; **P < 0.05. Scale bars = 150 μm.
Figure 4.
APP-processing, Aβ-production, or Aβ-degradation enzymes are not significantly altered in AAV1-mIL-6-expressing mice. A) Representative anti-CT20 immunoblot showed no significant changes in APP levels in AAV1-mIL-6-expressing P0 → 5 mo TgCRND8 compared with age-matched controls. B) Intensity analysis of anti-CT20 immunoreactive APP levels was normalized to β-actin in P0 → 5 mo TgCRND8 mouse cohort. C) Representative anti-CT20 immunoblot analysis of CTFα and CTFβ levels showed no significant changes in P0 → 5 mo TgCRND8 mice injected with AAV1-mIL-6 compared with age-matched controls. D). Intensity analysis of anti-CT20 immunoreactive CTF bands was normalized to β-actin in P0 → 5 mo TgCRND8 mouse cohort. E) Representative immunoblot showed no significant changes in APP levels in P0 → 5 mo mIL-6-expressing nontransgenic CRND8 littermates compared with age-matched controls. F). Intensity analysis of anti-CT20 immunoreactive APP levels was normalized to β-actin in P0 → 5 mo nontransgenic CRND8 mouse cohort. G) No change in diethylamine-soluble endogenous Aβ40 levels was seen in mIL-6-expressing P0 → 5 mo nontransgenic CRND8 littermates compared with age-matched controls. H) Representative immunoblot analysis of GFAP, ApoE, and BACE1 using RIPA-soluble lysates of P0 → 5 mo TgCRND8 mice showed minimal changes in ApoE or BACE1 levels (_n_=3/group), whereas GFAP reactivity was significantly increased in mIL-6-expressing mice. I) Intensity analysis of GFAP, ApoE, and BACE1 levels was quantified after normalization to β-actin levels in P0 → 5 mo TgCRND8 mouse cohort. *P < 0.05. J) Quantitative RT-PCR analysis of mRNA levels of Aβ degrading enzymes Neprilysin and IDE in mIL-6-expressing mouse forebrain. Data (fold change over control) represent average values obtained by quantitative PCR on 3-mo-old non-Tg CRND8 mice injected with AAV1-EGFP (control) or AAV1-mIL-6 on P0. Data reflect 2 independent experiments; n = 4 mice/group. *P < 0.001.
Figure 5.
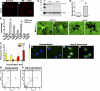
mIL6-induced persistent microglial up-regulation results in efficacious plaque clearance. A, B) Analysis of cd11b/Mac in mIL-6-expressing P0 → 5 mo TgCRND8 mice (_n_=3–4/group). Representative image showing up-regulation of cd11b/Mac in mIL-6-expressing TgCRND8 mice (B) compared with controls (A) detected by immunofluorescent staining on free-floating fixed sections. View ×200. C) Representative immunoblot analysis of cd11b in RIPA soluble brain extracts from mIL-6-injected P0 → 5 mo TgCRND8 mice compared with age-matched controls. D) Quantitative analysis of cd11b immunoblotting after normalizing to β-actin levels. *P < 0.05. E) Quantitative RT-PCR analysis of levels of microglial markers in mIL-6-expressing mouse forebrain. Data (fold change over control) represent average values obtained by QPCR on 3-mo-old mice injected with AAV1-EGFP (control) or AAV1-mIL-6 on P0; 2 independent experiments; n = 4 mice/group. *P < 0.001; 2-way ANOVA with Bonferroni’s posttests. F–I) Representative images of thioflavin-S-stained Aβ plaques (fluorescent green labeling) decorated with Iba-1 immunoreactive microglia (black immunostain) in mIL-6-expressing P0 → 5 mo TgCRND8 mice (H, I) and controls (F, G). View ×400. J) Quantitative analysis of the extent of Iba-1 immunodeposits circumscribing individual plaques shows increased association of activated microglia with plaques in mIL-6-expressing P0 → 5 mo TgCRND8 mice compared with controls (n = 4–5 mice/group). K–N) mIL-6-treated primary microglia appear to be more efficient in the uptake of fAβ42-Hilyte488 (green fluorescence, M, N) compared with unstimulated glia (K, L). Blue fluorescence indicates DAPI-stained glial nuclei. Data from 2 independent experiments; view ×600. O–P) Microglial cells with internalized Aβ42-Hilyte 488 (FITC channel on _x_-axis) were quantified by FACS. Percentage of positive cells in mIL-6 stimulated microglial cells was 10.2% (P, P3) compared with 4.2% in control unstimulated cells (O, P3).
Similar articles
- Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition.
Chakrabarty P, Tianbai L, Herring A, Ceballos-Diaz C, Das P, Golde TE. Chakrabarty P, et al. Mol Neurodegener. 2012 Jul 29;7:36. doi: 10.1186/1750-1326-7-36. Mol Neurodegener. 2012. PMID: 22838967 Free PMC article. - Human Alzheimer's disease gene expression signatures and immune profile in APP mouse models: a discrete transcriptomic view of Aβ plaque pathology.
Rothman SM, Tanis KQ, Gandhi P, Malkov V, Marcus J, Pearson M, Stevens R, Gilliland J, Ware C, Mahadomrongkul V, O'Loughlin E, Zeballos G, Smith R, Howell BJ, Klappenbach J, Kennedy M, Mirescu C. Rothman SM, et al. J Neuroinflammation. 2018 Sep 6;15(1):256. doi: 10.1186/s12974-018-1265-7. J Neuroinflammation. 2018. PMID: 30189875 Free PMC article. - IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice.
Chakrabarty P, Ceballos-Diaz C, Beccard A, Janus C, Dickson D, Golde TE, Das P. Chakrabarty P, et al. J Immunol. 2010 May 1;184(9):5333-43. doi: 10.4049/jimmunol.0903382. Epub 2010 Apr 5. J Immunol. 2010. PMID: 20368278 Free PMC article. - The neuroprotective N-terminal amyloid-β core hexapeptide reverses reactive gliosis and gliotoxicity in Alzheimer's disease pathology models.
Lantz MJ, Roberts AM, Delgado DD, Nichols RA. Lantz MJ, et al. J Neuroinflammation. 2023 May 27;20(1):129. doi: 10.1186/s12974-023-02807-9. J Neuroinflammation. 2023. PMID: 37245024 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
- Modulating innate immune activation states impacts the efficacy of specific Aβ immunotherapy.
Levites Y, Funk C, Wang X, Chakrabarty P, McFarland KN, Bramblett B, O'Neal V, Liu X, Ladd T, Robinson M, Allen M, Carrasquillo MM, Dickson D, Cruz P, Ryu D, Li HD, Price ND, Ertekin-Taner N, Golde TE. Levites Y, et al. Mol Neurodegener. 2021 May 6;16(1):32. doi: 10.1186/s13024-021-00453-4. Mol Neurodegener. 2021. PMID: 33957936 Free PMC article. - West Nile Virus-Induced Neurologic Sequelae-Relationship to Neurodegenerative Cascades and Dementias.
Vittor AY, Long M, Chakrabarty P, Aycock L, Kollu V, DeKosky ST. Vittor AY, et al. Curr Trop Med Rep. 2020 Mar;7(1):25-36. doi: 10.1007/s40475-020-00200-7. Epub 2020 Feb 18. Curr Trop Med Rep. 2020. PMID: 32775145 Free PMC article. - Neuroinflammation in Alzheimer's disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation.
Hensley K. Hensley K. J Alzheimers Dis. 2010;21(1):1-14. doi: 10.3233/JAD-2010-1414. J Alzheimers Dis. 2010. PMID: 20182045 Free PMC article. Review. - Animal models in the study of Alzheimer's disease and Parkinson's disease: A historical perspective.
Banerjee R, Rai A, Iyer SM, Narwal S, Tare M. Banerjee R, et al. Animal Model Exp Med. 2022 Feb;5(1):27-37. doi: 10.1002/ame2.12209. Epub 2022 Jan 27. Animal Model Exp Med. 2022. PMID: 35229999 Free PMC article. Review. - Time course of focused ultrasound effects on β-amyloid plaque pathology in the TgCRND8 mouse model of Alzheimer's disease.
Poon CT, Shah K, Lin C, Tse R, Kim KK, Mooney S, Aubert I, Stefanovic B, Hynynen K. Poon CT, et al. Sci Rep. 2018 Sep 19;8(1):14061. doi: 10.1038/s41598-018-32250-3. Sci Rep. 2018. PMID: 30232364 Free PMC article.
References
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G M, Cooper N R, Eikelenboom P, Emmerling M, Fiebich B L, Finch C E, Frautschy S, Griffin W S, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie I R, McGeer P L, O'Banion M K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel F L, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Ringheim G E, Szczepanik A M, Petko W, Burgher K L, Zhu S Z, Chao C C. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain Res Mol Brain Res. 1998;55:35–44. - PubMed
- Gao H M, Jiang J, Wilson B, Zhang W, Hong J S, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AG71216/AG/NIA NIH HHS/United States
- R01 AG018454/AG/NIA NIH HHS/United States
- R01AG18454/AG/NIA NIH HHS/United States
- P01 AG025531/AG/NIA NIH HHS/United States
- R01AG29886/AG/NIA NIH HHS/United States
- R01 AG029886/AG/NIA NIH HHS/United States
- P01AG25531/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases