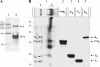The XcpV/GspI pseudopilin has a central role in the assembly of a quaternary complex within the T2SS pseudopilus - PubMed (original) (raw)
The XcpV/GspI pseudopilin has a central role in the assembly of a quaternary complex within the T2SS pseudopilus
Badreddine Douzi et al. J Biol Chem. 2009.
Abstract
Gram-negative bacteria use the sophisticated type II secretion system (T2SS) to secrete a large number of exoproteins into the extracellular environment. Five proteins of the T2SS, the pseudopilins GspG-H-I-J-K, are proposed to assemble into a pseudopilus involved in the extrusion of the substrate through the outer membrane channel. Recent structural data have suggested that the three pseudopilins GspI-J-K are organized in a trimeric complex located at the tip of the GspG-containing pseudopilus. In the present work we combined two biochemical techniques to investigate the protein-protein interaction network between the five Pseudomonas aeruginosa Xcp pseudopilins. The soluble domains of XcpT-U-V-W-X (respectively homologous to GspG-H-I-J-K) were purified, and the interactions were tested by surface plasmon resonance and affinity co-purification in all possible combinations. We found an XcpV(I)-W(J)-X(K) complex, which demonstrates that the crystallized trimeric complex also exists in the P. aeruginosa T2SS. Interestingly, our systematic approach revealed an additional and yet uncharacterized interaction between XcpU(H) and XcpW(J). This observation suggested the existence of a quaternary, rather than ternary, complex (XcpU(H)-V(I)-W(J)-X(K)) at the tip of the pseudopilus. The assembly of this quaternary complex was further demonstrated by co-purification using affinity chromatography. Moreover, by testing various combinations of pseudopilins by surface plasmon resonance and affinity chromatography, we were able to dissect the different possible successive steps occurring during the formation of the quaternary complex. We propose a model in which XcpV(I) is the nucleator that first binds XcpX(K) and XcpW(J) at different sites. Then the ternary complex recruits XcpU(H) through a direct interaction with XcpW(J).
Figures
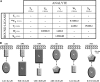
FIGURE 1.
Pseudopilin interaction network using surface plasmon resonance (BIAcore). A, each ligand was tested with the five analytes. The _K_diss values (μ
m
) for the interaction were detected. In parentheses are the chip used for each ligand and the molecular mass of each analyte. B, schemes for all positive interactions, with the _K_diss values indicated.
FIGURE 2.
Epitope mapping and binary and ternary interaction of pseudopilin soluble domains using surface plasmon resonance (BIAcore). A, WJ (5 μ
m
) and binary mix WJ-XK, (5 μ
m
) were passed on VI bound to a CM5 chip. B, binding pattern of XK alone (20–1.25 μ
m
) on VI bound to a CM5 chip. D, binding pattern of binary mix WJ-XK (10–0.62 μ
m
) on VI bound to a CM5 chip. E, binding pattern of WJ-XK-UH mix (10–0.31 μ
m
) on VI bound to a CM5 chip. A, B, D, and E, we report on the y axis the variation of plasmon resonance in arbitrary unit (ΔRU) and the reaction time on x axis. C and F, schemes of the WJ-VI-XK and UH-WJ-VI-XK complexes proposed, with _K_diss of the interaction and _k_on and _k_off values when calculable.
FIGURE 3.
Batch co-purification of pseudopilin-soluble domains on affinity column. Each of the His6-tagged protein was mixed with different untagged protein partners to dissect the pseudopilin quaternary complex. WJ-UH interaction is shown in A; the requirements of VI for the formation of WJ-VI-XK ternary and UH-WJ-VI-XK quaternary complexes are presented in B and C, respectively; E and F demonstrate that TG does not integrate the UH-WJ-VI-XK quaternary complex. After affinity co-purification of proteins bound to the Ni2+-NTA-magnetic beads, the collected fractions were analyzed on a 15% SDS-PAGE. After electrophoresis, the gel was stained with Coomassie Blue. Fractions L1, L2, and L, respectively, contain the His6-tagged protein, the untagged protein partners, or both tagged and untagged proteins. Fraction FT contains the flow-through, fraction W contains the final wash, and fraction E contains the eluate. The positions of molecular mass markers are indicated on the left side of each gel (kDa). The positions of the various pseudopilin periplasmic domains are indicated on the right side of each gel or lane. In D, when the samples were run on a 12% SDS-PAGE for a longer time, the hisUH and WJ bands could be distinguished (surrounded with a frame), but under these conditions, VI runs out of the gel (not shown). The presence of hisUH and WJ in the doublet band (lane E) was confirmed by mass spectrometry. The faint band present just below HisWJ in the presence or not of other pseudopilins corresponds to a HisWJ degradation product, as confirmed by mass spectrometry analysis.
FIGURE 4.
Direct evidence of the quaternary complex Xcp hisWJ-UH-VI-XK. A, native 8–16% Tris-HCl PAGE (Bio-Rad) showing the migration of a native low molecular mass marker (lane 1) and the quaternary complex eluted from the co-purification presented in Fig. 3_C_ (panel 1, lane E). The position of the two major, “a” and “b” native complexes are indicated. B, second dimension: 15% SDS-PAGE showing the composition of the complex isolated from the Native-PAGE. Lane 1 contains the entire lane 2 from A that has been cut off, boiled, and placed horizontally in the slot (see “Experimental Procedures”). Lanes 2-5 contain purified hisWJ, UH, VI, and XK, respectively. The positions of the pseudopilins periplasmic domains are indicated on the right and reveal the presence of four of them in the quaternary complex (a) and of XcphisWJand XcpUH in the binary complex (b). Denaturated low molecular mass markers are shown on the left of the gel.
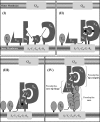
FIGURE 5.
Interaction network among Xcp pseudopilins and model for pseudopilus assembly. A schematic representation of the Xcp T2SS of P. aeruginosa is proposed. The inner membrane plate form, composed of XcpSF, -YL, -ZM, -PC, and -RE and the outer membrane secretin XcpQD are shown as light gray rectangles. The minor pseudopilins, XcpUH, -VI, -WJ, and -XK are represented by differently shaped forms (dark gray) that illustrate the complementarity of their interaction interfaces. For instance, XcpVI can interact with both XcpWJ and XcpXK, with two different interaction sites. The black asterisk indicates that the interaction involves the periplasmic domains of the pseudopilins. The major pseudopilin XcpTG is shown as an oval shape (medium gray) that can interact with itself during pseudopilus assembly, as well as with XcpUH. The white asterisk indicates that these interactions involve the transmembrane domains of the pseudopilins. We proposed the following ordered series of events leading to the assembly of the pseudopilus: Panel I, XcpVI enters first the inner membrane plateform and then recruits both XcpWJ and XcpXK to form the pseudopilus tip complex. Panel II, XcpUH then enters the ternary complex XcpVI-WJ-XK via its interaction with XcpWJ. Panel III, the tip quaternary complex is then able to accommodate the major pseudopilin XcpTG via a “hydrophobic” interaction with XcpUH. Panel IV, further polymerization of XcpTG pseudopilins triggers pseudopilus growth, with XcpUH fulfilling a core-tip hinge function between the pseudopilus core and tip.
Similar articles
- The type II secretion system: biogenesis, molecular architecture and mechanism.
Korotkov KV, Sandkvist M, Hol WG. Korotkov KV, et al. Nat Rev Microbiol. 2012 Apr 2;10(5):336-51. doi: 10.1038/nrmicro2762. Nat Rev Microbiol. 2012. PMID: 22466878 Free PMC article. Review. - Structure of the minor pseudopilin XcpW from the Pseudomonas aeruginosa type II secretion system.
Franz LP, Douzi B, Durand E, Dyer DH, Voulhoux R, Forest KT. Franz LP, et al. Acta Crystallogr D Biol Crystallogr. 2011 Feb;67(Pt 2):124-30. doi: 10.1107/S0907444910051954. Epub 2011 Jan 15. Acta Crystallogr D Biol Crystallogr. 2011. PMID: 21245534 Free PMC article. - Structure-guided disruption of the pseudopilus tip complex inhibits the Type II secretion in Pseudomonas aeruginosa.
Zhang Y, Faucher F, Zhang W, Wang S, Neville N, Poole K, Zheng J, Jia Z. Zhang Y, et al. PLoS Pathog. 2018 Oct 22;14(10):e1007343. doi: 10.1371/journal.ppat.1007343. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30346996 Free PMC article. - XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus.
Durand E, Michel G, Voulhoux R, Kürner J, Bernadac A, Filloux A. Durand E, et al. J Biol Chem. 2005 Sep 9;280(36):31378-89. doi: 10.1074/jbc.M505812200. Epub 2005 Jul 12. J Biol Chem. 2005. PMID: 16012171 - The structure and mechanism of the bacterial type II secretion system.
Naskar S, Hohl M, Tassinari M, Low HH. Naskar S, et al. Mol Microbiol. 2021 Mar;115(3):412-424. doi: 10.1111/mmi.14664. Epub 2020 Dec 29. Mol Microbiol. 2021. PMID: 33283907 Review.
Cited by
- Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin.
Nguyen Y, Sugiman-Marangos S, Harvey H, Bell SD, Charlton CL, Junop MS, Burrows LL. Nguyen Y, et al. J Biol Chem. 2015 Jan 2;290(1):601-11. doi: 10.1074/jbc.M114.616904. Epub 2014 Nov 11. J Biol Chem. 2015. PMID: 25389296 Free PMC article. - The type II secretion system: biogenesis, molecular architecture and mechanism.
Korotkov KV, Sandkvist M, Hol WG. Korotkov KV, et al. Nat Rev Microbiol. 2012 Apr 2;10(5):336-51. doi: 10.1038/nrmicro2762. Nat Rev Microbiol. 2012. PMID: 22466878 Free PMC article. Review. - Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation.
Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. Cisneros DA, et al. EMBO J. 2012 Feb 15;31(4):1041-53. doi: 10.1038/emboj.2011.454. Epub 2011 Dec 9. EMBO J. 2012. PMID: 22157749 Free PMC article. - The Role of Minor Pilins in Assembly and Function of the Competence Pilus of Streptococcus pneumoniae.
Oliveira V, Aschtgen MS, van Erp A, Henriques-Normark B, Muschiol S. Oliveira V, et al. Front Cell Infect Microbiol. 2021 Dec 22;11:808601. doi: 10.3389/fcimb.2021.808601. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004361 Free PMC article. - Direct interactions between the secreted effector and the T2SS components GspL and GspM reveal a new effector-sensing step during type 2 secretion.
Michel-Souzy S, Douzi B, Cadoret F, Raynaud C, Quinton L, Ball G, Voulhoux R. Michel-Souzy S, et al. J Biol Chem. 2018 Dec 14;293(50):19441-19450. doi: 10.1074/jbc.RA117.001127. Epub 2018 Oct 18. J Biol Chem. 2018. PMID: 30337370 Free PMC article.
References
- Filloux A. (2004) Biochim. Biophys. Acta. 1694, 163–179 - PubMed
- Pelicic V. (2008) Mol. Microbiol. 68, 827–837 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources