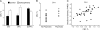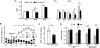How high-resolution basal-state functional imaging can guide the development of new pharmacotherapies for schizophrenia - PubMed (original) (raw)
How high-resolution basal-state functional imaging can guide the development of new pharmacotherapies for schizophrenia
Inna Gaisler-Salomon et al. Schizophr Bull. 2009 Nov.
Abstract
We describe here a coordinated brain imaging and animal models approach in which we have shown that the hippocampal CA1 region is a principal node in schizophrenia pathogenesis and have identified a novel treatment approach to the disorder based on inhibition of glutamate release. To identify biomarkers, we have focused on the putative prodromal period, typically lasting a few years, preceding the first onset of psychosis. About one-third of a high-risk cohort followed prospectively for 2.5 years will progress to threshold psychosis, making it possible to perform a relatively short prospective study. We have utilized a technological development in functional imaging techniques in which we measure cerebral blood volume (CBV), which allows for interrogation of subregions of the brain in the basal state at submillimeter resolution. Measurements of CBV in schizophrenia as well as in high-risk or prodromal stages can then pinpoint brain subregions differentially targeted during the earliest stages of the disorder. Our data suggest that the CA1 subfield of the hippocampal formation is most consistently implicated across disease stages, identifying a putative biomarker suitable for guiding drug development. Our studies in transgenic mice mutant in the glutamate synthetic enzyme glutaminase support the hypothesis that CA1 hyperfunction is due to altered glutamatergic neurotransmission. As a proof of principle, the glutaminase-deficient mice suggest that pharmacotherapies that reduce glutamatergic neurotransmission in the CA1 subfield may be a uniquely effective therapeutic strategy in schizophrenia and preventative in prodromal stages of the disorder.
Figures
Fig. 1.
Clinical CBV Imaging. (A) An increase in CBV between control and schizophrenia groups was observed selectively in hippocampal CA1 and the orbitofrontal cortex (OFC), while a CBV decrease was observed in the dorsolateral prefrontal cortex (DLPFC). (B) Initial baseline CBV measurements in CA1 in the prodromal subjects who progressed clinically to psychosis (after 2 y) were significantly higher than those who did not. (C) CA1 CBV correlated with delusional severity (measured on the positive and negative symptom scale, PANSS). Source: Redrawn from Schobel et al, with permission of the American Medical Association (Archives of General Psychiatry). Copyright 2009 American Medical Association. All rights reserved. CBV, cerebral blood volume; CA1, hippocampal subregion.
Fig. 2.
CBV Imaging of the Hippocampus in Clinical Subjects and Mice. (A) In clinical subjects (A1), high-resolution T1-weighted images in a coronal section can resolve hippocampal subregions (A2). Individual CBV maps of the hippocampal formation are shown from a healthy control (A3), a prodromal subject (A4), and a schizophrenia subject (A5). Maps are color coded such that warmer colors reflect higher CBV values. CBV increased from the control to the high-risk subject in CA1 and to the schizophrenia subject in CA1 and SUB. (B) In mouse, high-resolution images in a horizontal section (B1) resolve hippocampal subregions (B2). In WT mice, regional CBV is fairly uniform across hippocampal subregions (B3), while in GLS1 het mice, CA1 and SUB show a selective reduction (B4). A similar comparison in FC revealed no difference between WT and GLS1 het mice (images not shown). CBV, cerebral blood volume, HIPP, hippocampus; CA1 and CA3, hippocampal subregions; SUB, subiculum; DG, dentate gyrus; THAL, thalamus; EC, entorhinal cortex; FC, frontal cortex; WT, wild-type mice; GLS1 het, GLS1 heterozygous mice. Source: Panel A is redrawn from Schobel et al, with permission of the American Medical Association (Archives of General Psychiatry). Copyright 2009 American Medical Association. All rights reserved. Panel B is redrawn from Gaisler-Salomon et al with permission of the authors.
Fig. 3.
Schizophrenia Resilience Phenotypes of GLS1 hets. (A) GLS1 hets have reduced rCBV in the HIPP but not in the FC or THAL; WT > GLS1 hets (A1). Within the HIPP subregions, rCBV was significantly reduced in CA1 and SUB (A2). WT > GLS1 hets, *P < .05, numbers per group are given in parentheses. (B) GLS1 het mice show an attenuated response to amphetamine (Amph). Ambulatory distance was measured prior to and after intraperitoneal injection of amphetamine (2 mg/kg) or saline (injection, arrow). WT mice showed a robust increase in activity following Amph, while GLS1 hets, as well as saline-treated mice of both genotypes, showed no increase in activity. WT-Amph > GLS1 het-Amph, GLS1 het-saline, WT-saline, *P < .05. GLS1 hets do respond to higher-dose Amph. (C) While microdialysis measurements showed no genotypic difference in baseline DA recovery, the DA surge following Amph was attenuated in the GLS1 hets. WT-Amph > GLS1-Amph, *P < .05. (D) GLS1 hets displayed clozapine (Cloz)-like potentiation of LI. Low freezing levels were observed in WT and GLS1 het mice under saline- or Cloz-treated conditions prior to testing. Freezing ratios during the tone test showed LI. LIis seen as a lower suppression ratio in pre-exposed (PE) compared with not pre-exposed (NPE) subjects. We observed LI in Cloz-treated WT mice and in saline-and Cloz-treated GLS1 hets but not in saline-treated WT mice. NPE > PE, *P < .05, **P < .01. Cloz-treated GLS1 hets did not show enhanced LI. Thus, GLS1 het mice appear similar to WT mice treated with Cloz. GLS1 het, GLS1 heterozygous mice; HIPP, hippocampus; FC, frontal cortex; THAL, thalamus; WT, wild-type mice; CA1 and CA3, hippocampal subregion; SUB, subiculum; DG, dentate gyrus; EC, entorhinal cortex; RCBV, regional CBV; LI, latent inhibition. Source: Redrawn from Gaisler-Salomon et al with permission of the authors.
Similar articles
- Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia.
Gaisler-Salomon I, Miller GM, Chuhma N, Lee S, Zhang H, Ghoddoussi F, Lewandowski N, Fairhurst S, Wang Y, Conjard-Duplany A, Masson J, Balsam P, Hen R, Arancio O, Galloway MP, Moore HM, Small SA, Rayport S. Gaisler-Salomon I, et al. Neuropsychopharmacology. 2009 Sep;34(10):2305-22. doi: 10.1038/npp.2009.58. Epub 2009 Jun 10. Neuropsychopharmacology. 2009. PMID: 19516252 Free PMC article. - Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Schobel SA, et al. Arch Gen Psychiatry. 2009 Sep;66(9):938-46. doi: 10.1001/archgenpsychiatry.2009.115. Arch Gen Psychiatry. 2009. PMID: 19736350 Free PMC article. - Glutamate Dehydrogenase-Deficient Mice Display Schizophrenia-Like Behavioral Abnormalities and CA1-Specific Hippocampal Dysfunction.
Lander SS, Khan U, Lewandowski N, Chakraborty D, Provenzano FA, Mingote S, Chornyy S, Frigerio F, Maechler P, Kaphzan H, Small SA, Rayport S, Gaisler-Salomon I. Lander SS, et al. Schizophr Bull. 2019 Jan 1;45(1):127-137. doi: 10.1093/schbul/sby011. Schizophr Bull. 2019. PMID: 29471549 Free PMC article. - Is dopamine neurotransmission altered in prodromal schizophrenia? A review of the evidence.
Bauer M, Praschak-Rieder N, Kasper S, Willeit M. Bauer M, et al. Curr Pharm Des. 2012;18(12):1568-79. doi: 10.2174/138161212799958611. Curr Pharm Des. 2012. PMID: 22280434 Review. - Schizophrenia and glutamatergic transmission.
Tamminga CA. Tamminga CA. Crit Rev Neurobiol. 1998;12(1-2):21-36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. Crit Rev Neurobiol. 1998. PMID: 9444480 Review.
Cited by
- The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions.
Baharnoori M, Bartholomeusz C, Boucher AA, Buchy L, Chaddock C, Chiliza B, Föcking M, Fornito A, Gallego JA, Hori H, Huf G, Jabbar GA, Kang SH, El Kissi Y, Merchán-Naranjo J, Modinos G, Abdel-Fadeel NA, Neubeck AK, Ng HP, Novak G, Owolabi OO, Prata DP, Rao NP, Riecansky I, Smith DC, Souza RP, Thienel R, Trotman HD, Uchida H, Woodberry KA, O'Shea A, DeLisi LE. Baharnoori M, et al. Schizophr Res. 2010 Dec;124(1-3):e1-62. doi: 10.1016/j.schres.2010.09.008. Epub 2010 Oct 8. Schizophr Res. 2010. PMID: 20934307 Free PMC article. - Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat.
Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S, Bifone A, Zucconi GG, Bentivoglio M. Gozzi A, et al. Neuropsychopharmacology. 2012 Feb;37(3):822-37. doi: 10.1038/npp.2011.260. Epub 2011 Nov 2. Neuropsychopharmacology. 2012. PMID: 22048464 Free PMC article. - Synaptic underpinnings of altered hippocampal function in glutaminase-deficient mice during maturation.
Gaisler-Salomon I, Wang Y, Chuhma N, Zhang H, Golumbic YN, Mihali A, Arancio O, Sibille E, Rayport S. Gaisler-Salomon I, et al. Hippocampus. 2012 May;22(5):1027-39. doi: 10.1002/hipo.22014. Epub 2012 Mar 19. Hippocampus. 2012. PMID: 22431402 Free PMC article. - Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration.
Shirai Y, Fujita Y, Hashimoto K. Shirai Y, et al. Clin Psychopharmacol Neurosci. 2012 Aug;10(2):94-8. doi: 10.9758/cpn.2012.10.2.94. Epub 2012 Aug 31. Clin Psychopharmacol Neurosci. 2012. PMID: 23430731 Free PMC article. - Reduced limbic metabolism and fronto-cortical volume in rats vulnerable to alcohol addiction.
Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A. Gozzi A, et al. Neuroimage. 2013 Apr 1;69:112-9. doi: 10.1016/j.neuroimage.2012.12.015. Epub 2012 Dec 20. Neuroimage. 2013. PMID: 23261637 Free PMC article.
References
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. - PubMed
- Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Annu Rev Med. 2007;58:49–61. - PubMed
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. - PubMed
- Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull. 1997;23:385–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous