Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity - PubMed (original) (raw)
Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity
Enikö A Kramár et al. J Neurosci. 2009.
Abstract
Estrogen, in addition to its genomic effects in brain, causes rapid and reversible changes to synaptic operations. We report here that these acute actions are due to selective activation of an actin-signaling cascade normally used in the production of long-term potentiation (LTP). Estrogen, or a selective agonist of the steroid's beta-receptor, caused a modest increase in fast glutamatergic transmission and a pronounced facilitation of LTP in adult hippocampal slices; both effects were completely eliminated by latrunculin, a toxin that prevents actin filament assembly. Estrogen also increased spine concentrations of filamentous actin and strongly enhanced its polymerization in association with LTP. A search for the origins of these effects showed that estrogen activates the small GTPase RhoA and phosphorylates (inactivates) the actin severing protein cofilin, a downstream target of RhoA. Moreover, an antagonist of RhoA kinase (ROCK) blocked estrogen's synaptic effects. Estrogen thus emerges as a positive modulator of a RhoA>ROCK>LIM kinase>cofilin pathway that regulates the subsynaptic cytoskeleton. It does not, however, strongly affect a second LTP-related pathway, involving the GTPases Rac and Cdc42 and their effector p21-activated kinase, which may explain why its acute effects are reversible. Finally, ovariectomy depressed RhoA activity, spine cytoskeletal plasticity, and LTP, whereas brief infusions of estrogen rescued plasticity, suggesting that the deficits in plasticity arise from acute, as well as genomic, consequences of hormone loss.
Figures
Figure 1.
Estrogen receptor stimulation reversibly increases synaptic transmission. Field EPSPs (fEPSPs) elicited by stimulation of the Schaffer–commissural projections were recorded in CA1b stratum radiatum of slices prepared from the hippocampus of adult male rats. A, Infusion of estradiol (E2) at concentrations of 0.01 (n = 3), 0.1 (n = 4), and 1 (n = 4) n
m
caused a rapid, dose-dependent, and reversible increase in fEPSP slope (infusion period indicated by bar). Inset, Traces collected during baseline and at the end of E2 infusion (calibration: 1 mV, 5 ms). B, The effects of E2 were reproduced by 20 min infusions of WAY (1–100 n
m
, n = 3–5), a selective agonist for estrogen's β receptors. An agonist for the α receptors of estrogen (PPT: 100 n
m
, n = 3) had no evident effects on synaptic responses. Inset, Traces collected during baseline and at peak response to 100 n
m
WAY (calibration: 1 mV/5 ms). C, The graph shows the percentage (mean ± SEM) paired-pulse facilitation (ppf) of the initial slope of fEPSPs expressed as a function of the interpulse interval (40, 60, 100, and 200 ms) for responses recorded before (dotted lines) and during (solid lines) infusion of 1 n
m
estradiol (n = 5) or the selective agonist 100 n
m
WAY (n = 5). Neither treatment significantly influenced paired-pulse facilitation.
Figure 2.
Estradiol selectively facilitates AMPA receptor-mediated components of fast EPSPs. A, Pharmacologically isolated IPSPs (responses recorded in the presence of 20 μ
m
DNQX and 100 μ
m
APV, antagonists for AMPA and NMDA receptors, respectively) were elicited by stimulation of the Schaffer–commissural projections. These responses were completely blocked by the GABAA receptor antagonist picrotoxin (PTX, upper right traces) and were unaffected by infusions of 1 n
m
E2 (upper left traces; also see text). Paired-pulse depression (200 ms interstimulus interval), a characteristic feature of fast IPSPs (see text), was unaffected by E2 (upper left traces and graph); subsequent infusion of the presynaptic GABAB receptor antagonist saclofen (100 μ
m
) (upper right traces and graph) confirmed that the depression was due to GABA autoreceptors (n = 5). Calibration: 0.1 mV, 50 ms. B, The NMDA receptor component of the fEPSP was isolated by incubating the slices with 20 μ
m
DNQX and 100 μ
m
PTX. The resultant evoked potentials were unaffected by 20 min infusions of 1 n
m
E2 and were eliminated by APV (n = 4). The traces included in the inset illustrate the slow kinetics of the isolated NMDA response (calibration: 0.1 mV, 20 ms) and the extent to which it is insensitive to E2. C, E2 (1 n
m
) enhances isolated AMPA-receptor-mediated fEPSPs. The graph summarizes the results for a group of slices (n = 4) tested in the presence of APV and PTX. E2 (1 n
m
) caused an ∼20% increase in the amplitude of the isolated AMPA receptor-mediated fEPSP, a value comparable to that obtained in untreated slices (see Fig. 1). The traces included in the inset illustrate the E2 effect and the extent to which the responses were eliminated by the AMPA receptor antagonist DNQX.
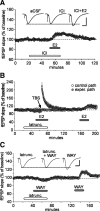
Figure 3.
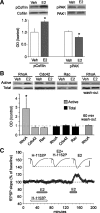
Effects of estrogen receptor stimulation on the threshold and ceiling for LTP in field CA1. A, Two or three theta bursts (arrows) did not reliably change the fEPSP slope in hippocampal slices (male rats) continuously infused with vehicle (0.01% DMSO) or the ER-α agonist PPT, but produced stable potentiation following pretreatment with estradiol (E2) or the ER-β agonist WAY. Five or ten theta bursts (arrows) generated greater potentiation in E2- or WAY-treated slices than in vehicle- or PPT-infused slices (n = 5/group). The upper dashed line denotes maximum LTP during control (aCSF) conditions. Inset: Traces collected during baseline recording (solid line) and 30 min after delivery of 3, 5 or 10 theta bursts (dotted lines) from slices infused with vehicle, E2 or WAY. Calibration: 1 mV, 5 ms. B, Percentage increase in burst response area across a series of 10 theta bursts in vehicle, PPT, WAY, and E2-treated slices; within-train facilitation was not significantly different between groups. C, Traces show the composite responses generated by a pair of theta bursts (200 ms between-burst interval) recorded in the presence and absence of 100 μ
m
APV; percentage change in the areas of the fourth EPSP (red hash marks) provides a measure of NMDA receptor-mediated depolarization (calibration: 1 mV, 10 ms). As described in text, E2 did not affect this component of burst responses used to induce LTP (n = 4).
Figure 4.
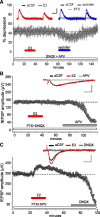
Latrunculin blocks the effects of estradiol on synaptic physiology. Responses to Schaffer–commissural stimulation were recorded from field CA1b of slices (from male rats) treated with reagents during periods indicated by the horizontal bars. A, Treatment with ICI-182,780 (ICI, 100 μ
m
) to block estradiol's nuclear receptor did not reduce E2-induced increases in fEPSP slope (n = 5). Inset: representative traces collected during baseline, ICI-182,780 infusion and at peak 1 n
m
E2 response. Calibration: 1 mV, 5 ms. B, Pretreatment with 500 n
m
latrunculin (latrunc.) blocked the effects of E2 on fEPSPs elicited by a collection of Schaffer–commissural projections stimulated at 0.05 Hz stimulation throughout the experiment (open circles). Ten theta bursts delivered to a second population of afferents produced a robust short-term potentiation that rapidly decayed back to baseline (closed circles). A 90 min period of latrunculin washout restored responsiveness of the fEPSP to 1 n
m
E2. C, Infusions of 500 n
m
latrunculin blocked, in a fully reversible manner, the increase in baseline responses produced by the ER-β agonist WAY (100 n
m
; n = 6). Inset, Representative traces collected during periods of treatment with latrunculin, WAY, and both. Calibration 1 mV, 5 ms.
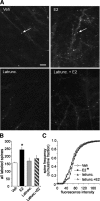
Figure 5.
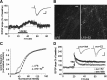
Estradiol promotes actin polymerization in dendritic spines. A, The top two panels compare phalloidin labeling in slices treated with 1 n
m
E2 or vehicle; the increase in spine-like profiles (arrows) produced by the steroid is typical of what is found in survey micrographs. The bottom two panels illustrate the effects of latrunculin (500 n
m
) alone on baseline and in combination with E2-induced labeling. Scale bar, 5 μm. B, The group mean (±SEM) number of dendritic spines with phalloidin labeling was significantly greater in E2 slices (n = 21 slices) compared with vehicle controls (n = 14 slices; p = 0.0005). E2 did not produce a significant effect in slices that had been pretreated with latrunculin. C, Cumulative frequency distribution of phalloidin-labeling intensities in CA1b str. radiatum in control versus E2-treated slices; the steroid shifted the distribution significantly to the right (i.e., toward more intense labeling) relative to the control profile (p < 0.001, Kruskal–Wallis test). This effect was blocked by latrunculin.
Figure 6.
E2 enhances theta burst-induced LTP and actin polymerization. A, E2 (1 n
m
) or vehicle was infused for 20 min. and then washed out for 60 min. The steroid produced its typical ∼20%, reversible increase in the slope of the fEPSPs elicited in field CA1 by single pulse stimulation of the Schaffer–commissural fibers. A single train of three theta bursts delivered at the end of vehicle infusion caused a robust short-term potentiation that decayed back to baseline over the following 60 min. The same train applied at the end of E2 infusion generated robust and stable LTP (33 ± 9% at 60 min after infusion; p = 0.005 vs TBS in vehicle-treated slices). B, Rhodamine-labeled phalloidin was applied at the end of the 60 min washout period and the slices processed for epifluorescence microscopy. Survey micrographs indicated that slices receiving LFS during E2 treatment (“E2-LFS”) did not increase the number of labeled spines above levels seen in vehicle-treated cases (“Veh-LFS”). Three theta bursts also appeared to be without effect when applied in the presence of vehicle (“Veh+TBS”) but caused an evident increase in the numbers of labeled spines when delivered at the end of E2 infusion (“E2+TBS”). Scale bar, 5 μm. C, An automated system was used to count the number of spines associated with dense phalloidin labeling in slices collected 60 min after the various treatments. Slices that received only LFS had the same, low number of labeled spines whether treated with E2 or vehicle. Three theta bursts did not affect the frequency of phalloidin-positive spines when delivered to vehicle-treated slices but caused a pronounced increase when administered 60 min earlier in the presence of E2 (*p < 0.0001 vs E2 without TBS) (n = 8 slices/group).
Figure 7.
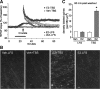
Estradiol selectively activates a RhoA-to-actin signaling pathway. Hippocampal slices (male rats) were treated with vehicle (Veh) or 1 n
m
estradiol (E2) for 20 min. A, Representative Western blots show at left levels of phosphorylated (p) cofilin and total cofilin and at right levels of pPAK and total PAK1 in hippocampal slices treated with vehicle or E2. Quantitative analyses (n = 6 slices/group) confirmed that E2 selectively increased levels of pCofilin relative to total cofilin (bar graph; p < 0.05 for E2 vs Veh), whereas E2 modestly but significantly reduced pPAK relative to measures from vehicle controls (n = 8/group; p < 0.01). Total PAK levels were unaffected. B, Total and active (GTP-bound) levels of RhoA, Cdc42, and Rac in vehicle- and E2-treated slices as seen in Western blots of material collected with the pull-down assay. Plots of band densities (bottom panel) indicate that E2 increased slice concentrations of activated RhoA (n = 3–5/group; p < 0.05 vs Veh control) while having no effect on total RhoA. Results from similar experiments detected no effect of E2 on active or total levels of the related GTPases Rac and Cdc42. Following a 60 min washout of E2, activated RhoA levels were not significantly different from those in vehicle-treated slices (n = 3/group). C, The ROCK inhibitor H-1152P (250 n
m
; open horizontal bar) reversibly blocked the increase in synaptic responses induced by E2 (n = 4). Inset, Representative traces collected during treatment with H-1152P, and E2 alone and in combination. Calibration: 1 mV, 5 ms.
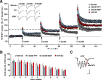
Figure 8.
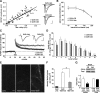
Ovariectomy disrupts actin signaling and synaptic plasticity in CA1. Hippocampal slices were prepared from middle-aged (9–10 months old) rats that had been ovariectomized (OVX) for 6 months before receiving either estrogen replacement (17β-estradiol benzoate or EB; 10 μg/0.1 ml, n = 8) or vehicle injections (Veh, sesame oil; n = 8) every other day for 2 weeks. A, Input/output curves compare amplitudes of the presynaptic fiber volley to the fEPSP. Linear regression slopes were not detectably different at any current tested (10, 20, 30, 40, and 50 μA) for slices prepared from OVX + Veh versus OVX + EB rats (n = 8 slices/group). Representative traces are shown at right (calibration: 1 mV, 5 ms). B, Paired-pulse facilitation of the initial slope of the synaptic response was comparable at all interpulse intervals (40, 60, 100, and 200 ms) in the two groups of slices. C, A train of 10 theta bursts produced stable potentiation in slices from OVX rats given EB but not from those given vehicle. Insets show fEPSP traces collected during baseline recording (solid line) and 30 min after TBS (dotted line). Calibration: 1 mV, 5 ms. D, The facilitation of theta burst responses within a train was comparable in slices prepared from OVX rats given EB or vehicle. The data are expressed as the percentage increase in the areas of the numbered burst response over the area of the first burst response (n = 8/group). E, Survey micrographs of phalloidin labeling in slices prepared from OVX rats. Slices from OVX + Veh or OVX + EB rats had few labeled spines following LFS. TBS (10 bursts) produced what appeared to be a modest increase in labeling in OVX + Veh slices and a pronounced increase in OVX + EB slices. Scale bar, 5 μm. F, Numbers of phalloidin-labeled profiles measured with an automated counting program. Estimates for the LFS condition were comparable in slices prepared from OVX + Veh and OVX + EB rats (3 ± 2 and 2 ± 3 spines per 550 μm2, respectively) and the groups are combined in the graph (OVX + LFS, n = 10 slices). The TBS-induced increase in numbers of labeled puncta was significantly greater in slices from OVX + EB (p = 0.0007; n = 9 slices) than in OVX + Veh (n = 7 slices) rats. G, Representative Western blots of activated and total RhoA (as determined in pull-down assays) and bar graph showing quantification of active and total RhoA bands from four experiments. Levels of active RhoA were significantly lower in hippocampal tissue collected from OVX + Veh rats than in OVX + EB rats (p = 0.03).
Figure 9.
Acute infusions of estradiol offset the effects of ovariectomy on actin dynamics and synaptic plasticity. A, E2 (1 n
m
) infusions produced a rapid and reversible increase in synaptic responses collected from OVX slices (n = 6) comparable in magnitude to that found in slices from young adult rats (see Fig. 1). Inset: traces collected during baseline (solid line) and at peak response to infusion of E2 (dotted line). Calibration: 0.5 mV, 5 ms. B, Twenty minute infusions of 1 n
m
E2 increased phalloidin labeling of spine heads in OVX slices relative to vehicle-treated cases. Scale bar, 5 μm. C, E2 caused a significant rightward shift in the intensity versus frequency distribution for phalloidin labeling (p < 0.001, Kruskal–Wallis test). D, The impairment in LTP (10 theta bursts) found in slices from OVX rats was offset by infusion of 1 n
m
E2 (horizontal bar); percentage LTP at 60 min after TBS was 49 ± 12% for E2-treated (n = 5) and 19 ± 8% for vehicle (Veh)-treated (n = 5; p = 0.002) slices. Inset: fEPSP traces collected during baseline (solid line) and at 60 min after TBS (dotted line). Calibration: 1 mV, 5 ms.
Similar articles
- Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton.
Kramár EA, Babayan AH, Gall CM, Lynch G. Kramár EA, et al. Neuroscience. 2013 Jun 3;239:3-16. doi: 10.1016/j.neuroscience.2012.10.038. Epub 2012 Oct 25. Neuroscience. 2013. PMID: 23103216 Free PMC article. Review. - ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity.
Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. Gu J, et al. Nat Neurosci. 2010 Oct;13(10):1208-15. doi: 10.1038/nn.2634. Epub 2010 Sep 12. Nat Neurosci. 2010. PMID: 20835250 Free PMC article. - Estrogen's Place in the Family of Synaptic Modulators.
Kramár EA, Chen LY, Rex CS, Gall CM, Lynch G. Kramár EA, et al. Mol Cell Pharmacol. 2009 Jan 1;1(5):258-262. Mol Cell Pharmacol. 2009. PMID: 20419049 Free PMC article. - An activity-regulated microRNA, miR-188, controls dendritic plasticity and synaptic transmission by downregulating neuropilin-2.
Lee K, Kim JH, Kwon OB, An K, Ryu J, Cho K, Suh YH, Kim HS. Lee K, et al. J Neurosci. 2012 Apr 18;32(16):5678-5687. doi: 10.1523/JNEUROSCI.6471-11.2012. J Neurosci. 2012. PMID: 22514329 Free PMC article. - Rapid effects of oestrogen on synaptic plasticity: interactions with actin and its signalling proteins.
Babayan AH, Kramár EA. Babayan AH, et al. J Neuroendocrinol. 2013 Nov;25(11):1163-72. doi: 10.1111/jne.12108. J Neuroendocrinol. 2013. PMID: 24112361 Free PMC article. Review.
Cited by
- The estrous cycle modulates hippocampal spine dynamics, dendritic processing, and spatial coding.
Wolcott NS, Redman WT, Karpinska M, Jacobs EG, Goard MJ. Wolcott NS, et al. bioRxiv [Preprint]. 2024 Aug 3:2024.08.02.606418. doi: 10.1101/2024.08.02.606418. bioRxiv. 2024. PMID: 39131375 Free PMC article. Preprint. - In Vitro Cell Culture Model for Osteoclast Activation during Estrogen Withdrawal.
Gandhi N, Omer S, Harrison RE. Gandhi N, et al. Int J Mol Sci. 2024 Jun 1;25(11):6134. doi: 10.3390/ijms25116134. Int J Mol Sci. 2024. PMID: 38892322 Free PMC article. - Mechanism of Microwave Radiation-Induced Learning and Memory Impairment Based on Hippocampal Metabolomics.
Guan S, Xin Y, Ren K, Wang H, Dong J, Wang H, Zhang J, Xu X, Yao B, Zhao L, Peng R. Guan S, et al. Brain Sci. 2024 Apr 29;14(5):441. doi: 10.3390/brainsci14050441. Brain Sci. 2024. PMID: 38790420 Free PMC article. - Altered brain morphology and functional connectivity in postmenopausal women: automatic segmentation of whole-brain and thalamic subnuclei and resting-state fMRI.
Kim GW, Park K, Kim YH, Jeong GW. Kim GW, et al. Aging (Albany NY). 2024 Mar 23;16(6):4965-4979. doi: 10.18632/aging.205662. Epub 2024 Mar 23. Aging (Albany NY). 2024. PMID: 38526330 Free PMC article. - Role of estrogen in sex differences in memory, emotion and neuropsychiatric disorders.
Iqbal J, Huang GD, Xue YX, Yang M, Jia XJ. Iqbal J, et al. Mol Biol Rep. 2024 Mar 12;51(1):415. doi: 10.1007/s11033-024-09374-z. Mol Biol Rep. 2024. PMID: 38472517 Review.
References
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. - PubMed
- Arai A, Silberg J, Lynch G. Differences in the refractory properties of two distinct inhibitory circuitries in field CA1 of the hippocampus. Brain Res. 1995;704:298–306. - PubMed
- Devi G, Hahn K, Massimi S, Zhivotovskaya E. Prevalence of memory loss complaints and other symptoms associated with the menopause transition: a community survey. Gend Med. 2005;2:255–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS045540/NS/NINDS NIH HHS/United States
- NS051923/NS/NINDS NIH HHS/United States
- P01 NS045260-05/NS/NINDS NIH HHS/United States
- F31 MH083396/MH/NIMH NIH HHS/United States
- R01 NS051823/NS/NINDS NIH HHS/United States
- MH083396/MH/NIMH NIH HHS/United States
- R01 NS051823-07/NS/NINDS NIH HHS/United States
- T32 NS045540/NS/NINDS NIH HHS/United States
- NS045260/NS/NINDS NIH HHS/United States
- P01 NS045260/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous