Stem cell therapies benefit Alport syndrome - PubMed (original) (raw)
Stem cell therapies benefit Alport syndrome
Valerie LeBleu et al. J Am Soc Nephrol. 2009 Nov.
Abstract
Patients with Alport syndrome progressively lose renal function as a result of defective type IV collagen in their glomerular basement membrane. In mice lacking the alpha3 chain of type IV collagen (Col4A3 knockout mice), a model for Alport syndrome, transplantation of wild-type bone marrow repairs the renal disease. It is unknown whether cell-based therapies that do not require transplantation have similar potential. Here, infusion of wild-type bone marrow-derived cells into unconditioned, nonirradiated Col4A3 knockout mice during the late stage of disease significantly improved renal histology and function. Furthermore, transfusion of unfractionated wild-type blood into unconditioned, nonirradiated Col4A3 knockout mice improved the renal phenotype and significantly improved survival. Injection of mouse and human embryonic stem cells into Col4A3 knockout mice produced similar results. Regardless of treatment modality, the improvement in the architecture of the glomerular basement membrane is associated with de novo expression of the alpha3(IV) chain. These data provide further support for testing cell-based therapies for Alport syndrome.
Figures
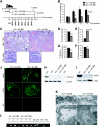
Figure 1.
BM cell infusions in C57BL/6 Col4A3 knockout mice rescue the renal phenotype. (A) Schematic representation of experimental setup: BM infusions were administered to seven Col4A3 knockout littermates with end-stage renal failure (20 wk of age), with four Col4A3 knockout mice receiving WT BM from GFP+ donor and three Col4A3 knockout mice receiving Col4A3 knockout BM. Recipients were treated with seven consecutive BM infusions and sacrificed at 23.5 wk of age after one of the mice in the experimental group of Col4A3 knockout mice infused at 20 weeks with BM transplant from Col4A3 knockout died. (B) Urine albumin-creatinine ratio measurements in Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice (n = 3) and mice that received BM transplant from WT mice (n = 4) from 20 to 23 wk of age. (C) Representative hematoxylin and eosin (H&E) staining of the kidney cortex of 23.5-wk-old mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice; black arrowheads point to globally sclerosed glomeruli, and green arrowheads point to normal healthy glomeruli. Representative periodic acid-Schiff staining of glomeruli. (D through G) Morphometric analyses of percentage of tubular atrophy (D), percentage of normal glomeruli (E), percentage of glomerular global sclerosis (F), and percentage of interstitial volume of 23.5 wk-old Col4A3 knockout mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice (G). (H) RT-PCR analyses for expression of Col4A3 knockout and β-actin control. (I) Western blot immunolabeling for mouse α3(IV) and mouse α5(IV) from ECM proteins from kidneys of WT, Col4A3 knockout mice that received BM transplant from Col4A3 knockout, and Col4A3 knockout mice that received BM transplant from WT mice. (J) Immunolabeling of mouse α3(IV) and α5(IV) collagen in kidney glomeruli of Col4A3 knockout mice that received BM transplant from Col4A3 knockout and mice that received BM transplant from WT mice mice; secondary FITC-conjugated antibody was used in negative control (NC). (K) Representative transmission electron microscopy images from Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice and mice that received BM transplant from WT mice at 23.5 wk of age. P, podocyte; FP, foot processes. GBM is partly underlined. (L) Genotyping PCR amplification of Col4a3 WT (1000-bp product) and Col4A3 knockout (850-bp product) gene from genomic DNA extracted from the BM of Col4A3 knockout mice that received BM transplant from Col4A3 knockout mice, Col4A3 knockout mice that received BM transplant from WT mice, Col4A3 knockout, WT, and Col4A3 heterozygote (HET). NC, no template control. *P < 0.05; **P < 0.01. Magnifications: ×100 and ×400 in C; ×400 in J.
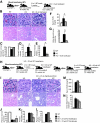
Figure 2.
Blood transfusions rescue Col4A3 knockout renal phenotype. (A) Schematic representation of experimental setup: WT donor mice were used for blood transfusion in 8-wk-old C57BL/6 Col4A3 knockout mice (n = 4). The mice were subsequently killed at 12.5 wk of age. (B through D) Representative H&E pictures at higher (top) and lower (bottom) magnifications of kidneys from WT (n = 3), Col4A3 knockout (n = 6), and Col4A3 knockout mice transfused at 8 wk(n = 4) (B) and morphometric analyses of percentage of normal glomeruli (C), percentage of interstitial volume (D), and percentage of tubular atrophy (E). (F and G) BUN measurements (F) and urine albumin-creatinine ratio (G) of 12.5-wk-old WT (n = 6), Col4A3 knockout (n = 3), and Col4A3 knockout mice transfused at 8 wk(n = 4). (H) Schematic representation of experimental setup: WT and Col4A3 knockout donor mice were used for blood transfusion in 23.5-wk-old Col4A3 knockout mice ([n = 3] and [n = 7], respectively). (I through L) Representative H&E pictures at higher (top) and lower (bottom) magnifications of kidneys from 21-wk-old WT (n = 3) and Col4A3 knockout (n = 3), 25-wk-old Col4A3 knockout transfused at 23 wk with Col4A3 knockout blood (n = 3), and 27-wk-old Col4A3 knockout transfused at 23 wk with WT blood (n = 7) mice (I) and morphometric analyses of percentage of normal glomeruli (J), percentage of interstitial volume (K), and percentage of tubular atrophy (L). (M and N) BUN measurements (M) and urine albumin-creatinine ratio (N) from 21 wk-old WT (n = 3) and Col4A3 knockout (n = 3), 25-wk-old Col4A3 knockout transfused at 23 wk with Col4A3 knockout blood (n = 3), and Col4A3 knockout mice transfused at 23 wk with WT blood (n = 6) mice. *P < 0.05. Magnifications: ×400 in B (top) and I (top); ×200 in B (bottom) and I (bottom).
Figure 3.
Type IV collagen expression and restored GBM architecture are shown in Col4A3 knockout mice that received blood transfusions at 8 wk. (A, left) Western blot analysis reveals α3(IV) chain expression in type IV collagen hexamer (H) of WT and transfused Col4A3 knockout mice, but no expression is observed in Col4A3 knockout control mice. After denaturation of the ECM preparation, type IV collagen α3 dimers (D) and α3 and α4 monomers (M) could be detected in WT and transfused Col4A3 knockout mice but not in Col4A3 knockout mice. (Right) Immunoprecipitation of α3-, α4-, and α5-containing NC1 hexamers from collagenase-solubilized GBM by α3 antibody. Anti-α3 antibody was used to immunoprecipitate α3NC1-containing hexamers from collagenase solubilized GBM from WT and KO Blood 8 WT mice. SDS-PAGE resolves the immunoprecipitated α3NC1 hexamers into NC1 monomers and dimers. Western blot analyses reveal that α3, α4, and α5 NC1 monomers and dimers co-precipitate with α3 NC1-containing hexamers in both WT and transfused Col4A3 knockout mice, indicating the reemergence of α4 and α5 chain expression in the type IV collagen NC1 hexamers at 12.5 wk of age. Δ, Degradation product. (B) Ultrastructural analysis of the GBM by transmission electron microscopy of 12.5-wk-old WT, Col4A3 knockout, and transfused Col4A3 knockout mice. (C) Fluorescence in situ hybridization for the mouse Y-chromosome. Y-chromosome labeling shows recruitment of male blood–derived cells to glomeruli from female transfused Col4A3 knockout mice (12.5 wk of age). Magnification, ×31,000 in B; ×400 in C.
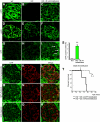
Figure 4.
Immunostaining and survival curve are shown for Col4A3 knockout mice that received a transfusion. (A through F) Type IV collagen α3 and α5 chain expression in the GBM of kidneys from WT, Col4A3 knockout, and Col4A3 knockout mice transfused at 8 wk with WT blood. No α3 chain is detected in the GBM of Col4A3 knockout control mice, and we detected faint α5 chain expression in Col4A3 knockout mice (B and E). Patchy α3 and α5 chain expression is detected in the GBM of Col4A3 knockout mice transfused at 8 wk with WT blood (C and F), similar to α3 and α5 chain expression in WT mice (A and D). (G through I) GFP labeling of kidney glomeruli: Whereas WT (G) and Col4A3 knockout (H) mice did not show any GFP labeling, Col4A3 knockout mice transfused at 8 wk with WT blood (transfused with whole blood from wild GFP+ mice) revealed GFP+ labeling in the glomeruli (I). (J through R) Co-localization of GFP labeling in glomeruli from Col4A3 knockout mice transfused at 8 wk with WT blood with the podocyte-specific markers synaptopodin (J through L), nephrin (P through R), and podocin (M through O). All tissue analyzed were from 12.5-wk-old mice. (S) Quantification of GFP+ cells revealed 3.3 ± 0.1% GFP+ cells per glomerulus at 12.5 wk of age in Col4A3 knockout mice transfused at 8 wk with WT blood (n = 3) and 0.2 ± 0.1% GFP+ cells per glomerulus at 12.5 wk of age in WT control mice (n = 3). (T) Survival curve depicts statistically significant increase in survival in Col4A3 knockout mice transfused at 23 wk with blood from WT mice (n = 7) in comparison with Col4A3 knockout mice transfused at 23 wk with blood from Col4A3 knockout mice (n = 3). T, termination, animals were killed. *P < 0.05; **P < 0.01. Magnification, ×400.
Comment in
- Cell therapy for Alport syndrome.
Wong CJ, Rogers I. Wong CJ, et al. J Am Soc Nephrol. 2009 Nov;20(11):2279-81. doi: 10.1681/ASN.2009090915. Epub 2009 Oct 9. J Am Soc Nephrol. 2009. PMID: 19820123 No abstract available.
Similar articles
- Endothelial cell-specific collagen type IV-α3 expression does not rescue Alport syndrome in Col4a3-/- mice.
Funk SD, Bayer RH, Miner JH. Funk SD, et al. Am J Physiol Renal Physiol. 2019 May 1;316(5):F830-F837. doi: 10.1152/ajprenal.00556.2018. Epub 2019 Feb 6. Am J Physiol Renal Physiol. 2019. PMID: 30724107 Free PMC article. - Irradiation prolongs survival of Alport mice.
Katayama K, Kawano M, Naito I, Ishikawa H, Sado Y, Asakawa N, Murata T, Oosugi K, Kiyohara M, Ishikawa E, Ito M, Nomura S. Katayama K, et al. J Am Soc Nephrol. 2008 Sep;19(9):1692-700. doi: 10.1681/ASN.2007070829. Epub 2008 May 14. J Am Soc Nephrol. 2008. PMID: 18480315 Free PMC article. - A human-mouse chimera of the alpha3alpha4alpha5(IV) collagen protomer rescues the renal phenotype in Col4a3-/- Alport mice.
Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC. Heidet L, et al. Am J Pathol. 2003 Oct;163(4):1633-44. doi: 10.1016/s0002-9440(10)63520-1. Am J Pathol. 2003. PMID: 14507670 Free PMC article. - Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome.
Cosgrove D, Liu S. Cosgrove D, et al. Matrix Biol. 2017 Jan;57-58:45-54. doi: 10.1016/j.matbio.2016.08.005. Epub 2016 Aug 27. Matrix Biol. 2017. PMID: 27576055 Free PMC article. Review. - Searching for a treatment for Alport syndrome using mouse models.
Katayama K, Nomura S, Tryggvason K, Ito M. Katayama K, et al. World J Nephrol. 2014 Nov 6;3(4):230-6. doi: 10.5527/wjn.v3.i4.230. World J Nephrol. 2014. PMID: 25374816 Free PMC article. Review.
Cited by
- Transplantation of umbilical cord mesenchymal stem cells into mice with focal segmental glomerulosclerosis delayed disease manifestation.
Shi Y, Xie J, Yang M, Ma J, Ren H. Shi Y, et al. Ann Transl Med. 2019 Aug;7(16):383. doi: 10.21037/atm.2019.07.71. Ann Transl Med. 2019. PMID: 31555697 Free PMC article. - Spotlight on Genetic Kidney Diseases: A Call for Drug Delivery and Nanomedicine Solutions.
Trac N, Ashraf A, Giblin J, Prakash S, Mitragotri S, Chung EJ. Trac N, et al. ACS Nano. 2023 Apr 11;17(7):6165-6177. doi: 10.1021/acsnano.2c12140. Epub 2023 Mar 29. ACS Nano. 2023. PMID: 36988207 Free PMC article. Review. - Stem cells as a therapeutic approach to chronic kidney diseases.
Sedrakyan S, Angelow S, De Filippo RE, Perin L. Sedrakyan S, et al. Curr Urol Rep. 2012 Feb;13(1):47-54. doi: 10.1007/s11934-011-0230-0. Curr Urol Rep. 2012. PMID: 22127675 Review. - In vivo directed differentiation of pluripotent stem cells for skeletal regeneration.
Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, Sun N, Lee M, Grova M, Connolly AJ, Wu JC, Gurtner GC, Weissman IL, Wan DC, Longaker MT. Levi B, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20379-84. doi: 10.1073/pnas.1218052109. Epub 2012 Nov 20. Proc Natl Acad Sci U S A. 2012. PMID: 23169671 Free PMC article. - Origin and function of myofibroblasts in kidney fibrosis.
LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. LeBleu VS, et al. Nat Med. 2013 Aug;19(8):1047-53. doi: 10.1038/nm.3218. Epub 2013 Jun 30. Nat Med. 2013. PMID: 23817022 Free PMC article.
References
- Kashtan CE: Alport syndrome: An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 78: 338–360, 1999 - PubMed
- Kalluri R, Cosgrove D: Assembly of type IV collagen: Insights from alpha3(IV) collagen-deficient mice. J Biol Chem 275: 12719–12724, 2000 - PubMed
- Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: Structure, gene organization, and role in human diseases—Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 - PubMed
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources