Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni - PubMed (original) (raw)
Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni
Barbro Lindmark et al. BMC Microbiol. 2009.
Abstract
Background: Background: Cytolethal distending toxin (CDT) is one of the well-characterized virulence factors of Campylobacter jejuni, but it is unknown how CDT becomes surface-exposed or is released from the bacterium to the surrounding environment.
Results: Our data suggest that CDT is secreted to the bacterial culture supernatant via outer membrane vesicles (OMVs) released from the bacteria. All three subunits (the CdtA, CdtB, and CdtC proteins) were detected by immunogold labeling and electron microscopy of OMVs. Subcellular fractionation of the bacteria indicated that, apart from the majority of CDT detected in the cytoplasmic compartment, appreciable amounts (20-50%) of the cellular pool of CDT proteins were present in the periplasmic compartment. In the bacterial culture supernatant, we found that a majority of the extracellular CDT was tightly associated with the OMVs. Isolated OMVs could exert the cell distending effects typical of CDT on a human intestinal cell line, indicating that CDT is present there in a biologically active form.
Conclusion: Our results strongly suggest that the release of outer membrane vesicles is functioning as a route of C. jejuni to deliver all the subunits of CDT toxin (CdtA, CdtB, and CdtC) to the surrounding environment, including infected host tissue.
Figures
Figure 1
Surface structure analyses of C. jejuni. Atomic force micrographs of (A) a C. jejuni strain 81-176 cell (Bar: 1 μm) and of (B) small and large OMVs (examples indicated by arrows) on the surface of a C. jeuni cell (Bar: 100 nm). (C) Electron micrograph of OMVs (examples indicated by arrows) isolated from C. jejuni strain 81-176 (Bar: 100 nm).
Figure 2
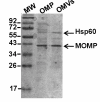
Protein profile of C. jejuni outer membrane and OMVs. Comparison of protein composition between the outer membrane protein fraction (OMP) and the OMVs sample from wild type C. jejuni strain 81-176. Protein bands were visualized by Coomassie blue staining of a SDS-PAGE gel.
Figure 3
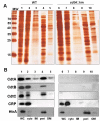
Immunoblot detection of intra- and extra-cellular CDT of C. jejuni. Immunoblot analyses of samples from C. jejuni wild type strains 81-176 (lanes 1-4) and the cdtA::km mutant (lanes 5-8). Samples: 1&5; whole cells (WC), 2&6; supernatants 1 (S1), 3&7; supernatants 2(S2), 4&8; OMVs, (A) Immunoblot detection with anti-CdtA polyclonal antiserum, (B) immunodetection with anti-CdtB polyclonal antiserum. (C) immunoblot detection with anti-CdtC polyclonal antiserum. (D) immunoblot detection with anti-Omp50 polyclonal antiserum.
Figure 4
Electron microscopy and immunogold labelling of CDT. Immunoelectron microscopic analyses of OMVs from wild type C. jejuni strain. 81-176 (A-C) and the cdtA::km mutant (D-F) using anti-CdtA (A, D), anti-CdtB (B, E), and anti-CdtC antisera (C, F). Arrows show the gold particles associated with OMVs. The square in the upper right corners show enlargements of parts of the micrographs. Bars correspond to 100 nm.
Figure 5
Electron microscopy and immunogold labelling of Hsp and Omp50. Immunoelectron microscopic analyses of OMVs. (A) OMVs of wild type C. jejuni strain 81-176 without antiserum (control). (B), immunolabelling with anti-Hsp antiserum. (C) immunolabelling with anti-Omp50 antiserum. White arrows show the GroEL like particles (in A) and the localization of gold particles on the GroEL like particles (in B). Black arrows show the OMVs (in A&B). Bars correspond to 100 nm.
Figure 6
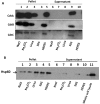
Analyses of CDT localization in subcellular C. jejuni fractions. Subcellular localization of CDT subunits in C. jejuni strain 81-176. (A), SDS-PAGE gel after silver staining and (B), immunoblot analyses of cell fractions from C. jejuni wild type strain 81-176 (lanes 1-5) and the cdtA::km mutant (lanes 6-10). Lanes 1&6, whole cell lysates; Lanes 2&7, cytoplasmic fractions 3 & 8, inner membrane fractions; Lanes 4 & 9, periplasmic fractions; Lanes 5 & 10, outer membrane fractions. Antisera for detection of CdtA, CdtB, CdtC, CRP, and HtrA, respectively, were used for the immunoblot analyses and representative results of repeated experiments are shown. Molecular weight markers are shown in the lane (MW) on the left. See materials and methods for details about the relative amount of the extracts used.
Figure 7
Analyses of CDT dissociation from OMVs. (A) Dissociation assays of CDT proteins associated with OMVs from C. jejuni. Samples of vesicles in 50 mM HEPES were treated for 60 min on ice in the presence of: NaCl (1 M), Na2CO3 (0.1 M), urea (8 M) or SDS 1%, respectively. The samples were then centrifuged and the resulting pellets (lanes 1-5) and supernatants (lanes 6-10) were analysed by SDS-PAGE and immunoblot analyses with anti-CdtA, anti-CdtB, and anti-CdtC antisera. (B) Dissociation assays of Hsp60 protein. Samples were treated as in (A), and the immunoblot analysis was done with anti-Hsp60 antiserum.
Figure 8
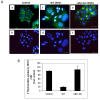
Analyses of biological activities of CDT. (A) Cytolethal distending effect by OMVs on HCT8 cells. HCT8 cells without treatment (a, b), HCT8 cells treated with 50 μl of OMVs (total protein concentration was 60 μg/ml) from wild type C. jejuni strain 81-176 (c, d), or from the cdtA::km mutant (e, f). After 72 hours of treatment the actin filaments and nuclei were stained with phalloidin and DAPI, respectively, as described in materials and methods. Upper panels (a, c, e) show merged images from staining with both dyes and lower panels (b, d, f) show images from DAPI staining only. Bars represent 40 μm. (B) Effect of thymidine uptake on HCT8 cells after treatment with OMVs from wild type C. jejuni strain 81-176 and the cdt::km mutant strain DS104 for 48 h. Cells were grown in 96-well plates and 10 μl of OMVs were added to the wells. The results are from triplicate wells and two independent experiments. Data are expressed as mean percentage (± SE).
Similar articles
- Campylobacter jejuni extracellular vesicles harboring cytolethal distending toxin bind host cell glycans and induce cell cycle arrest in host cells.
Le LHM, Elgamoudi B, Colon N, Cramond A, Poly F, Ying L, Korolik V, Ferrero RL. Le LHM, et al. Microbiol Spectr. 2024 Mar 5;12(3):e0323223. doi: 10.1128/spectrum.03232-23. Epub 2024 Feb 6. Microbiol Spectr. 2024. PMID: 38319111 Free PMC article. - CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity.
Lara-Tejero M, Galán JE. Lara-Tejero M, et al. Infect Immun. 2001 Jul;69(7):4358-65. doi: 10.1128/IAI.69.7.4358-4365.2001. Infect Immun. 2001. PMID: 11401974 Free PMC article. - Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells.
Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NF, Manson E, Imrie L, Bajaj-Elliott M, Wren BW, Smith DG, Dorrell N. Elmi A, et al. Infect Immun. 2012 Dec;80(12):4089-98. doi: 10.1128/IAI.00161-12. Epub 2012 Sep 10. Infect Immun. 2012. PMID: 22966047 Free PMC article. - Molecular Mechanisms and Potential Clinical Applications of Campylobacter jejuni Cytolethal Distending Toxin.
Lai CK, Chen YA, Lin CJ, Lin HJ, Kao MC, Huang MZ, Lin YH, Chiang-Ni C, Chen CJ, Lo UG, Lin LC, Lin H, Hsieh JT, Lai CH. Lai CK, et al. Front Cell Infect Microbiol. 2016 Feb 9;6:9. doi: 10.3389/fcimb.2016.00009. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26904508 Free PMC article. Review. - In vivo virulence properties of bacterial cytolethal-distending toxin.
Ge Z, Schauer DB, Fox JG. Ge Z, et al. Cell Microbiol. 2008 Aug;10(8):1599-607. doi: 10.1111/j.1462-5822.2008.01173.x. Epub 2008 May 16. Cell Microbiol. 2008. PMID: 18489725 Review.
Cited by
- Revisiting bacterial cytolethal distending toxin structure and function.
Chen H, Ang CJ, Crowder MK, Brieher WM, Blanke SR. Chen H, et al. Front Cell Infect Microbiol. 2023 Nov 14;13:1289359. doi: 10.3389/fcimb.2023.1289359. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38035327 Free PMC article. - Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans.
Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, Wai SN, Oscarsson J. Rompikuntal PK, et al. Infect Immun. 2012 Jan;80(1):31-42. doi: 10.1128/IAI.06069-11. Epub 2011 Oct 24. Infect Immun. 2012. PMID: 22025516 Free PMC article. - Protein selection and export via outer membrane vesicles.
Bonnington KE, Kuehn MJ. Bonnington KE, et al. Biochim Biophys Acta. 2014 Aug;1843(8):1612-9. doi: 10.1016/j.bbamcr.2013.12.011. Epub 2013 Dec 24. Biochim Biophys Acta. 2014. PMID: 24370777 Free PMC article. Review. - Membrane vesicle-mediated release of bacterial RNA.
Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Sjöström AE, et al. Sci Rep. 2015 Oct 20;5:15329. doi: 10.1038/srep15329. Sci Rep. 2015. PMID: 26483327 Free PMC article. - Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells.
Maldonado R, Wei R, Kachlany SC, Kazi M, Balashova NV. Maldonado R, et al. Microb Pathog. 2011 Jul-Aug;51(1-2):22-30. doi: 10.1016/j.micpath.2011.03.005. Epub 2011 Apr 2. Microb Pathog. 2011. PMID: 21443941 Free PMC article.
References
- Sack DA, Lyke C, McLaughlin C, Suwanvanichkij V. Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. WHO/CDS/CSR/DRS. 2001;8:31–40.
- Ismaeel AY, Jamsheer AE, Yousif AQ, Al-Otaibi MA, Botta GA. Causative pathogens of severe diarrhea in children. Saudi Med J. 2002;23(9):1064–1069. - PubMed
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366(9497):1653–1666. - PubMed
- Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290(5490):354–357. - PubMed
- Bereswill S, Kist M. Recent developments in Campylobacter pathogenesis. Curr Opin Infect Dis. 2003;16(5):487–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources