Dynein antagonizes eg5 by crosslinking and sliding antiparallel microtubules - PubMed (original) (raw)
Dynein antagonizes eg5 by crosslinking and sliding antiparallel microtubules
Nick P Ferenz et al. Curr Biol. 2009.
Abstract
Mitotic spindle assembly requires the combined activity of various molecular motor proteins, including Eg5 and dynein. Together, these motors generate antagonistic forces during mammalian bipolar spindle assembly; what remains unknown, however, is how these motors are functionally coordinated such that antagonism is possible. Given that Eg5 generates an outward force by crosslinking and sliding apart antiparallel microtubules (MTs), we explored the possibility that dynein generates an inward force by likewise sliding antiparallel MTs. We reasoned that antiparallel overlap, and therefore the magnitude of a dynein-mediated force, would be inversely proportional to the initial distance between centrosomes. To capitalize on this relationship, we utilized a nocodazole washout assay to mimic spindle assembly. We found that Eg5 inhibition led to either monopolar or bipolar spindle formation, depending on whether centrosomes were initially separated by less than or greater than 5.5 microm, respectively. Mathematical modeling predicted this same spindle bistability in the absence of functional Eg5 and required dynein acting on antiparallel MTs to do so. Our results suggest that dynein functionally coordinates with Eg5 by crosslinking and sliding antiparallel MTs, a novel role for dynein within the framework of spindle assembly.
Figures
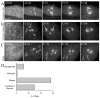
Figure 1. Spindle Assembly Following Nocodazole Washout
(A–C) Selected images from time-lapse sequences of cells treated with and released from nocodazole under the three centrosomal configurations. A, B and C correspond to Movies S2, S3 and S4, respectively. In each case, a bipolar spindle assembles following nocodazole washout. In the first image of each sequence, centrosomes appear as white dots. Arrows subsequently mark the position of in focus centrosomes when three or more foci are present. The last image of each sequence is a maximum intensity projection. All times are relative to the final nocodazole washout (0:00) and are displayed as min: sec. (D) Percentage of fixed LLC-Pk1α cells at the indicated mitotic stages, present 60min post-4x washout. Bar = 10μm.
Figure 2. Spindle Bistability in the Absence of Eg5 Activity
(A–C) Selected images from time-lapse sequences of cells treated with nocodazole and monastrol, then released into monastrol-containing medium. A and B correspond to Movies S5 and S6, respectively. Nocodazole washout leads to bipolar spindle formation, except when proximal centrosomes are close to one another. Set up is as defined in Figure 1. Additionally, asterisks mark the position of out of focus centrosomes. In C, two mitotic cells have fused together; the top spindle is acentrosomal. Bar = 10μm.
Figure 3. Dynein is Required for Monopolar Spindle Formation
(A–C) Selected images from time-lapse sequences of cells treated with nocodazole and monastrol, injected with p150-CC1, then released into monastrol-containing medium. A corresponds to Movie S7. In each case, a bipolar spindle assembles after nocodazole washout. Set up is as defined in Figure 2. Bar = 10μm.
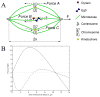
Figure 4. Mathematical Modeling
(A) Schematic of the mathematical model. The total force, F, acting on centrosomes is a function of Forces A, B and C (see text for descriptions). (B) Force versus intercentrosomal distance given by the model with a realistic chromosomal distribution at the spindle midplane (see Figure S6B) for L = 2; A = 1; C = 0.03 for uninhibited, co-inhibited (Eq. 4 in Supplemental Text, solid curve, B = 0) and Eg5-inhibited (Eq. 5 in Supplemental Text, dashed curve, B = 2) cells.
Similar articles
- Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly.
Tanenbaum ME, Macůrek L, Galjart N, Medema RH. Tanenbaum ME, et al. EMBO J. 2008 Dec 17;27(24):3235-45. doi: 10.1038/emboj.2008.242. Epub 2008 Nov 20. EMBO J. 2008. PMID: 19020519 Free PMC article. - The role of Hklp2 in the stabilization and maintenance of spindle bipolarity.
Vanneste D, Takagi M, Imamoto N, Vernos I. Vanneste D, et al. Curr Biol. 2009 Nov 3;19(20):1712-7. doi: 10.1016/j.cub.2009.09.019. Epub 2009 Oct 8. Curr Biol. 2009. PMID: 19818619 - The functional antagonism between Eg5 and dynein in spindle bipolarization is not compatible with a simple push-pull model.
Florian S, Mayer TU. Florian S, et al. Cell Rep. 2012 May 31;1(5):408-16. doi: 10.1016/j.celrep.2012.03.006. Epub 2012 Apr 20. Cell Rep. 2012. PMID: 22832270 - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian. - Molecular mechanisms in spindle positioning: structures and new concepts.
Stevermann L, Liakopoulos D. Stevermann L, et al. Curr Opin Cell Biol. 2012 Dec;24(6):816-24. doi: 10.1016/j.ceb.2012.10.005. Epub 2012 Nov 9. Curr Opin Cell Biol. 2012. PMID: 23142476 Review.
Cited by
- Spermatocytes have the capacity to segregate chromosomes despite centriole duplication failure.
Skinner MW, Simington CJ, López-Jiménez P, Baran KA, Xu J, Dayani Y, Pryzhkova MV, Page J, Gómez R, Holland AJ, Jordan PW. Skinner MW, et al. EMBO Rep. 2024 Aug;25(8):3373-3405. doi: 10.1038/s44319-024-00187-6. Epub 2024 Jun 28. EMBO Rep. 2024. PMID: 38943004 Free PMC article. - Kinetochore-mediated outward force promotes spindle pole separation in fission yeast.
Shirasugi Y, Sato M. Shirasugi Y, et al. Mol Biol Cell. 2019 Oct 15;30(22):2802-2813. doi: 10.1091/mbc.E19-07-0366. Epub 2019 Sep 18. Mol Biol Cell. 2019. PMID: 31532702 Free PMC article. - DnaJB6 is a RanGTP-regulated protein required for microtubule organization during mitosis.
Rosas-Salvans M, Scrofani J, Modol A, Vernos I. Rosas-Salvans M, et al. J Cell Sci. 2019 Jun 3;132(11):jcs227033. doi: 10.1242/jcs.227033. J Cell Sci. 2019. PMID: 31064815 Free PMC article. - An improved optical tweezers assay for measuring the force generation of single kinesin molecules.
Nicholas MP, Rao L, Gennerich A. Nicholas MP, et al. Methods Mol Biol. 2014;1136:171-246. doi: 10.1007/978-1-4939-0329-0_10. Methods Mol Biol. 2014. PMID: 24633799 Free PMC article. - Mitotic functions of kinesin-5.
Ferenz NP, Gable A, Wadsworth P. Ferenz NP, et al. Semin Cell Dev Biol. 2010 May;21(3):255-9. doi: 10.1016/j.semcdb.2010.01.019. Epub 2010 Jan 28. Semin Cell Dev Biol. 2010. PMID: 20109572 Free PMC article. Review.
References
- Blangy A, Lane HA, d’Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. - PubMed
- Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources