Imaging endocytic clathrin structures in living cells - PubMed (original) (raw)
Review
Imaging endocytic clathrin structures in living cells
Tom Kirchhausen. Trends Cell Biol. 2009 Nov.
Erratum in
- Trends Cell Biol. 2010 Jan;20(1):5
Abstract
Our understanding of the clathrin-dependent endocytic pathway owes much to new visualization techniques. Budding coated pits and clathrin-coated structures are transient molecular machines with distinctive morphological characteristics, and fluorescently labeled versions of a variety of marker proteins have given us a tantalizing glimpse of the dynamics of the system in living cells. Recent live-cell imaging studies have revealed unexpected modes of coat assembly, with distinct kinetics, distinct recruitment of associated proteins, distinct requirements for the participation of actin and its accessory proteins, and apparently distinct mechanisms of membrane deformation. A crucial issue is to connect the events detected by light microscopy with the structures and properties of the molecular constituents. Here, I outline descriptions of coat assembly in different circumstances that are consistent with what is known from X-ray crystallography and electron microscopy.
Figures
Figure 1
Formation of endocytic clathrin-coated structures on the plasma membrane of mammalian cells. The continuous line represents the plasma membrane of a cell grown in culture; a thick, ‘pink stripe’ represents the clathrin coat (clathrin plus the AP-2 adaptor); yellow dots represent dynamin; red dots, the uncoating ATP Hsc70 and its cofactor auxilin; short thin lines, short-branched actin polymers plus the Arp2/3 complex, cortactin and N-Wasp. The stippled thick pink stripe represents the ATP-dependent dissolution of the clathrin coat mediated by Hsc70 and auxilin. TIR, WF and spinning disk confocal microscopy have all been used to obtain live-cell imaging data; the vertical arrows indicate the _z_-positions accessible to each imaging method. Stable and transient expression of recombinant, fluorescently tagged constructs have been used to follow the dynamic behavior of plasma membrane structures containing different combinations of clathrin, AP-2, auxilin, Arp2/3 complex, cortactin and dynamin. Canonical clathrin-coated pits form at both free and attached cell surfaces; clathrin-coated plaques form only at the attached surface. Coated-pit formation proceeds by sequential addition of clathrin triskelions to an initial nucleus, generating a sharply curved coat; adaptor-mediated interactions with membrane-bound proteins (and lipids) deform the underlying membrane; dynamin mediates scission when the deformation has created a suitably narrow neck; auxilin, which arrives immediately following scission, recruits the uncoating ATPase, Hsc70. The relatively long-lasting clathrin-coated plaques probably initiate in a fashion similar to coated pits, but subsequent addition of clathrin triskelions maintains a relatively flat structure with mainly hexagonal facets. The number of clathrin triskelions and AP-2 complexes associated with a clathrin-coated plaque can fluctuate during its lifetime. Coated plaques drive membrane invagination when the entire coat moves rapidly inwards, in a step that requires formation of short-branched actin filaments; by contrast, actin polymerization does not generally accompany assembly or budding of coated pits. Abortive clathrin coats are incomplete, short-lived structures that fail to associate with cargo. Inset: diagram illustrating an older model, in which a flat coated plaque deforms into a sharply invaginated coated pit. Such a transformation is structurally implausible, as discussed in the text and illustrated by the exercise in Fig. 5.
Figure 2
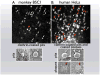
Different types of clathrin-coated structures detected on the attached surface of cells. The upper panels are single frames from time series recorded from: (a) a monkey BSC1 cell expressing σ2 EGFP of the adaptor AP-2; (b) a human HeLa cell expressing Tomato–LCa light chain of clathrin. The live-cell images are from optical sections, acquired with a spinning disk confocal microscope, at the level at which plasma membrane adheres to the glass coverslip. The white and red arrows single out short- and long-lived fluorescent objects, whose dynamic behavior corresponds to coated pits and coated plaques, respectively. The bottom panels are electron micrographs obtained from the attached surfaces of ‘unroofed’ preparations of the corresponding cell type. Whereas BSC1 cells display only deeply curved clathrin-coated structures (pits), HeLa cells contain both deeply curved and relatively flat clathrin lattices (pits and plaques, respectively). The clathrin lattices in coated pits contain both hexagonal and pentagonal facets; the coated plaques contain mostly hexagonal facets.
Figure 3
Morphology of clathrin-coated structures on the attached surface of cells. Electron micrograph obtained from an ‘unroofed’ preparation of human A4321 cells imaged by electron microscopy after quick-freeze deep etching, to show a range of different shapes of deeply curved and relatively flat clathrin structures on the adherent plasma membrane. (Reproduced, with permission, from .)
Figure 4
Receptor sorting and clathrin-coated pit formation. Schematic representation of a clathrin lattice during the assembly of a deeply invaginated coated pit, obtained from the accompanying animation, Movie 1. The shape and location of the triskelions are based on the map, of resolution 0.8 nm, of a clathrin coat obtained by cryoEM . The position of AP-2 within the coat is approximate. The shape of AP-2 is based on X-ray crystallographic data . AP-2 adaptor complexes bind selectively to the plasma membrane by interacting both with PtdIns(4,5)_P_2 in the membrane bilayer and with sorting motifs in the cytosolic tails of membrane-bound cargo receptors.
Figure 5
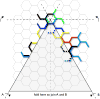
Rearrangements of clathrin triskelions in a flat hexagonal lattice that would be required to transform a single hexagon into a pentagon. This figure is adapted from . If hexagonal sheets are indeed intermediates in the budding of coated pits, major rearrangements of this kind would be necessary. High-resolution structural data show that a triskelion is centered at every vertex, with each of its three legs spanning three consecutive edges , . To simplify the drawing, only a subset of color-coded triskelions is shown, depicting just their proximal and distal legs (those spanning the two edges closest to the vertex). Thick lines highlight a subset of triskelions that would need to refold into the new lattice; the color-coded stippled lines indicate the new positions adopted by the legs after the rearrangement. The reader is invited to print the diagram (adapted from 60), to cut out the gray triangular region and to fold it along the lines indicated to generate a curved sheet. To make an approximately spherical clathrin lattice, 12 such rearrangements are required at precise locations within the lattice. This lattice contains 154 triskelions, and 72 would need to be removed (under the gray pie section) in order to transform a single hexagon into a pentagon. Eleven additional reductions (of different amounts, depending on the location of the new pentagons) are required to generate a completely enclosed structure. No such reductions have ever been detected during the formation of clathrin-coated structures. Clathrin is a relatively stiff protein (especially considering its contour length), with legs disposed at orientations relatively close to those adopted in the final coat . Overall, coat formation can readily be understood by sequential assembly of triskelions into a curved lattice. There is no need to postulate a flat, hexagonal intermediate, most of which would have to disassemble in order to generate curvature.
Similar articles
- Clathrin coat construction in endocytosis.
Pearse BM, Smith CJ, Owen DJ. Pearse BM, et al. Curr Opin Struct Biol. 2000 Apr;10(2):220-8. doi: 10.1016/s0959-440x(00)00071-3. Curr Opin Struct Biol. 2000. PMID: 10753805 Review. - Distinct dynamics of endocytic clathrin-coated pits and coated plaques.
Saffarian S, Cocucci E, Kirchhausen T. Saffarian S, et al. PLoS Biol. 2009 Sep;7(9):e1000191. doi: 10.1371/journal.pbio.1000191. Epub 2009 Sep 8. PLoS Biol. 2009. PMID: 19809571 Free PMC article. - Life of a clathrin coat: insights from clathrin and AP structures.
Edeling MA, Smith C, Owen D. Edeling MA, et al. Nat Rev Mol Cell Biol. 2006 Jan;7(1):32-44. doi: 10.1038/nrm1786. Nat Rev Mol Cell Biol. 2006. PMID: 16493411 Review. - ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat.
Avinoam O, Schorb M, Beese CJ, Briggs JA, Kaksonen M. Avinoam O, et al. Science. 2015 Jun 19;348(6241):1369-72. doi: 10.1126/science.aaa9555. Science. 2015. PMID: 26089517 - Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography.
Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BM. Musacchio A, et al. Mol Cell. 1999 Jun;3(6):761-70. doi: 10.1016/s1097-2765(01)80008-3. Mol Cell. 1999. PMID: 10394364
Cited by
- Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy.
Aguet F, Upadhyayula S, Gaudin R, Chou YY, Cocucci E, He K, Chen BC, Mosaliganti K, Pasham M, Skillern W, Legant WR, Liu TL, Findlay G, Marino E, Danuser G, Megason S, Betzig E, Kirchhausen T. Aguet F, et al. Mol Biol Cell. 2016 Nov 7;27(22):3418-3435. doi: 10.1091/mbc.E16-03-0164. Epub 2016 Aug 17. Mol Biol Cell. 2016. PMID: 27535432 Free PMC article. - Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes.
Hazan-Halevy I, Rosenblum D, Weinstein S, Bairey O, Raanani P, Peer D. Hazan-Halevy I, et al. Cancer Lett. 2015 Aug 1;364(1):59-69. doi: 10.1016/j.canlet.2015.04.026. Epub 2015 Apr 28. Cancer Lett. 2015. PMID: 25933830 Free PMC article. - Structure of the PTEN-like region of auxilin, a detector of clathrin-coated vesicle budding.
Guan R, Dai H, Harrison SC, Kirchhausen T. Guan R, et al. Structure. 2010 Sep 8;18(9):1191-8. doi: 10.1016/j.str.2010.06.016. Structure. 2010. PMID: 20826345 Free PMC article. - Clathrin- and Arp2/3-independent endocytosis in the fungal pathogen Candida albicans.
Epp E, Nazarova E, Regan H, Douglas LM, Konopka JB, Vogel J, Whiteway M. Epp E, et al. mBio. 2013 Aug 27;4(5):e00476-13. doi: 10.1128/mBio.00476-13. mBio. 2013. PMID: 23982070 Free PMC article. - Identification of a novel function of the clathrin-coated structure at the plasma membrane in facilitating GM-CSF receptor-mediated activation of JAK2.
Chen PH, Chien FC, Lee SP, Chan WE, Lin IH, Liu CS, Lee FJ, Lai JS, Chen P, Yang-Yen HF, Yen JJ. Chen PH, et al. Cell Cycle. 2012 Oct 1;11(19):3611-26. doi: 10.4161/cc.21920. Epub 2012 Aug 30. Cell Cycle. 2012. PMID: 22935703 Free PMC article.
References
- McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. - PubMed
- Kirchhausen T. Three ways to make a vesicle [Review] Nature Reviews Molecular Cell Biology. 2000;1:187–198. - PubMed
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. - PubMed
- Bonifacino JS, Traub LM. Signals for Sorting of Transmembrane Proteins to Endosomes and Lysosomes. Annu Rev Biochem 2003 - PubMed
- Hirst J, Robinson MS. Clathrin and adaptors. Biochem Biophys Acta. 1998;1404:173–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources