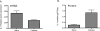A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats - PubMed (original) (raw)
A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats
Julie Wieseler et al. J Neurosci Methods. 2010.
Abstract
Migraine is a neurovascular disorder that induces debilitating headaches associated with multiple symptoms including facial allodynia, characterized by heightened responsivity to normally innocuous mechanical stimuli. It is now well accepted that immune activation and immune-derived inflammatory mediators enhance pain responsivity, including the trigeminal system. Nociceptive ("pain" responsive) trigeminal nerves densely innervate the cranial meninges. We have recently proposed that the meninges may serve as a previously unidentified, key interface between the peripheral immune system and the CNS with potential implications for understanding underlying migraine mechanisms. Our focus here is the development of a model for facial allodynia associated with migraine. We developed a model wherein an indwelling catheter is placed between the skull and dura, allowing immunogenic stimuli to be administered over the dura in awake and freely moving rats. Since the catheter does not contact the brain itself, any proinflammatory cytokines induced following manipulation derive from resident or recruited meningeal immune cells. While surgery alone does not alter immune activation markers, TNF or IL6 mRNA and/or protein, it does decrease gene expression and increase protein expression of IL-1 at 4 days after surgery. Using this model we show the induction of facial allodynia in response to supradural administration of either the HIV glycoprotein gp120 or inflammatory soup (bradykinin, histamine, serotonin, and prostaglandin E2), and the induction of hindpaw allodynia in our model after inflammatory soup. This model allows time- and dose-dependent assessment of the relationship between changes in meningeal inflammation and corresponding exaggerated pain behaviors.
Figures
Figure 1. Photograph of the supradural catheter implantation sites
Troughs are bilaterally drilled in the skull beginning 2-mm from midline. The troughs are approximately 4-mm long, becoming gradually deeper at the more caudal point. At this point the skull is pierced, while leaving the dura intact. The catheters, constructed from PE-10 tubing, are guided by the troughs and gently inserted bilaterally between the skull and dura mater, with the gently sloping troughs allowing the catheter to slip beneath the skull parallel to the dural surface so to avoid piercing the dura. The catheters are marked to indicate when each is inserted 4–6 mm.
Figure 2. Gene and Protein expression for IL-1 are differentially altered in response to surgery
A) IL-1 gene expression was lower compared naïve control tissues. B) IL-1 protein expression was elevated compared to naïve control tissues. Surgery significantly influences the proinflammatory state in the meninges.
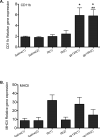
Figure 3. Gene expression is altered in response to saline, inflammatory soup and gp120
A) A single application of gp120 (gp120(1)) and double application of gp120 (gp120(2)) significantly up-regulated CD11b gene expression compared to single or double application of saline (saline (1) or saline (2); *), and compared to a single application of inflammatory soup (IS (1); **). B) MHCII expression was also affected, however the post hoc analyses did not reveal further significant differences. The graph suggests that single application of inflammatory soup (IS (1)) or gp120 (gp120(1)) up-regulates MHCII gene expression whereas saline, single or repeated application (saline (1) or saline (2)) does not alter MHCII.
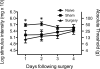
Figure 4. Facial allodynia induced by surgery is resolved 4 days post
Surgery for the placement of bilateral supradural catheters and sham surgery (skin incision with skull scraping) induced facial allodynia immediately following surgery on days 1 and 2. By day 4, behavior among surgery groups and naïve to surgery rats were indistinguishable. * indicates differences compared to naïve.
Figure 5. Mast cells are degranulated 4 days post supradural catheterization
A) Naïve meningeal tissues comparable to those surround the supradural catheter, and B) meningeal tissue surrounding the supradural catheter, stained with mast cell specific stain, toluidine blue.
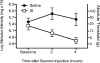
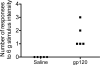
Figure 6. Development of facial allodynia in rats injected supradurally with gp120
Following baseline (pre-drug) assessment of response to calibrated von Frey filaments, rats received supradural injections of either saline or gp120. Two successive supradural injections of saline produced no reliable changes in behavior of awake and freely moving rats across the timecourse tested. In contrast, two successive supradural injections of gp120, separated by 2 hours, induced facial allodynia at 3 hours after the second injection.
Figure 7. Development of facial allodynia in rats injected supradurally with inflammatory soup (IS)
Following baseline (pre-drug) assessment of response to calibrated von Frey filaments, rats received supradural injections of either saline or IS. Two successive supradural injections of saline (filled circles) produced no reliable changes in the behavior of awake and freely moving rats across the timecourse tested. In contrast, two successive supradural injections of IS, separated by 2 hours, induced facial allodynia at 2 and 4 hours after the second injection.
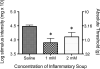
Figure 8. Development of hind-paw allodynia in rats injected supradurally with inflammatory soup (IS)
Following baseline (pre-drug) assessment of response to calibrated von Frey filaments, rats received 2 successive supradrual injections of saline, 1, or 2 mM IS. Based on facial allodynia time points, hind-paw allodynia was assessed 2 hours post the second injection. IS induced hind-paw allodynia at both 1 and 2 mM concentrations. * indicate differences from saline.
Similar articles
- Indwelling supradural catheters for induction of facial allodynia: surgical procedures, application of inflammatory stimuli, and behavioral testing.
Wieseler J, Sprunger D, Ellis A, Maier SF, Watkins LR. Wieseler J, et al. Methods Mol Biol. 2012;851:99-107. doi: 10.1007/978-1-61779-561-9_6. Methods Mol Biol. 2012. PMID: 22351084 Free PMC article. - Supradural inflammatory soup in awake and freely moving rats induces facial allodynia that is blocked by putative immune modulators.
Wieseler J, Ellis A, McFadden A, Stone K, Brown K, Cady S, Bastos LF, Sprunger D, Rezvani N, Johnson K, Rice KC, Maier SF, Watkins LR. Wieseler J, et al. Brain Res. 2017 Jun 1;1664:87-94. doi: 10.1016/j.brainres.2017.03.011. Epub 2017 Mar 16. Brain Res. 2017. PMID: 28322750 Free PMC article. - Medullary pain facilitating neurons mediate allodynia in headache-related pain.
Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Edelmayer RM, et al. Ann Neurol. 2009 Feb;65(2):184-93. doi: 10.1002/ana.21537. Ann Neurol. 2009. PMID: 19259966 Free PMC article. - Central sensitisation and cutaneous allodynia in migraine: implications for treatment.
Landy S, Rice K, Lobo B. Landy S, et al. CNS Drugs. 2004;18(6):337-42. doi: 10.2165/00023210-200418060-00001. CNS Drugs. 2004. PMID: 15089101 Review. - [The mechanism of peripheral and central sensitization in migraine. A literature review].
Tajti J, Vécsei L. Tajti J, et al. Neuropsychopharmacol Hung. 2009 Mar;11(1):15-21. Neuropsychopharmacol Hung. 2009. PMID: 19731814 Review. Hungarian.
Cited by
- Characterization of a mouse model of headache.
Huang D, Ren L, Qiu CS, Liu P, Peterson J, Yanagawa Y, Cao YQ. Huang D, et al. Pain. 2016 Aug;157(8):1744-1760. doi: 10.1097/j.pain.0000000000000578. Pain. 2016. PMID: 27058678 Free PMC article. - Activation of TRPA1 on dural afferents: a potential mechanism of headache pain.
Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Edelmayer RM, et al. Pain. 2012 Sep;153(9):1949-1958. doi: 10.1016/j.pain.2012.06.012. Epub 2012 Jul 17. Pain. 2012. PMID: 22809691 Free PMC article. - Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine.
Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Stucky NL, et al. Headache. 2011 May;51(5):674-92. doi: 10.1111/j.1526-4610.2011.01882.x. Headache. 2011. PMID: 21521205 Free PMC article. - The influence of rapid eye movement sleep deprivation on nociceptive transmission and the duration of facial allodynia in rats: a behavioral and Fos immunohistochemical study.
Kim SH, Park JY, Shin HE, Lee SB, Ryu DW, Kim TW, Park JW. Kim SH, et al. J Headache Pain. 2019 Mar 1;20(1):21. doi: 10.1186/s10194-019-0977-0. J Headache Pain. 2019. PMID: 30823867 Free PMC article. - Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache.
Burgos-Vega CC, Ahn DD, Bischoff C, Wang W, Horne D, Wang J, Gavva N, Dussor G. Burgos-Vega CC, et al. Cephalalgia. 2016 Feb;36(2):185-93. doi: 10.1177/0333102415584313. Epub 2015 May 5. Cephalalgia. 2016. PMID: 25944818 Free PMC article.
References
- Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–20. - PubMed
- Braun JS, Kaissling B, Le Hir M, Zenker W. Cellular components of the immune barrier in the spinal meninges and dorsal root ganglia of the normal rat: immunohistochemical (MHC class II) and electron-microscopic observations. Cell Tissue Res. 1993;273:209–17. - PubMed
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. - PubMed
- Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005;161:658–60. - PubMed
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 DA015642-05/DA/NIDA NIH HHS/United States
- DA015642/DA/NIDA NIH HHS/United States
- K02 DA015642/DA/NIDA NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
- R01 DE017782-04/DE/NIDCR NIH HHS/United States
- DA022042/DA/NIDA NIH HHS/United States
- DE017782/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical