A chemical platform for improved induction of human iPSCs - PubMed (original) (raw)
A chemical platform for improved induction of human iPSCs
Tongxiang Lin et al. Nat Methods. 2009 Nov.
Abstract
The slow kinetics and low efficiency of reprogramming methods to generate human induced pluripotent stem cells (iPSCs) impose major limitations on their utility in biomedical applications. Here we describe a chemical approach that dramatically improves (200-fold) the efficiency of iPSC generation from human fibroblasts, within seven days of treatment. This will provide a basis for developing safer, more efficient, nonviral methods for reprogramming human somatic cells.
Figures
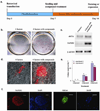
Figure 1. Compound treatment for seven days is sufficient to induce pluripotent stem cells from human fibroblasts transduced with the four reprogramming factors
(a) Timeline for human iPSC induction using combined SB431542 and PD0325901 treatment along with 4TFs. Treatment began with cell re-seeding at day 7 after 4TF transduction and was maintained for 7 days. (b) Staining for ALP+ colonies that emerged in the untreated (left) or 2 compound-treated (right) cultures within seven days. (c) RT-PCR showing elevated endogenous mRNA expression of pluripotency markers OCT4 and NANOG in 2 compound-treated cultures. (d) Tra-1-81 staining at day 14 without (left) or with (right) 2 compound treatment. (e) The numbers of NANOG+ colonies at day 14 under different treatment conditions are plotted. (f) Typical staining for hESC-specific markers (NANOG and SSEA4) exhibited by D14 iPSCs. Scale bars, 50 µm in (d & f)
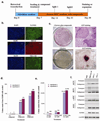
Figure 2. Prolonged compound treatment and cell passaging dramatically increased the number of reprogrammed colonies
(a) Timeline of human iPSC induction using SB431542, PD0325901 and thiazovivin. (b) Day 30 iPSCs expressed pluripotency markers NANOG, SSEA4 and TRA-1-81. Scale bars, 50 µm (c) ALP staining of day 30 cultures with (upper panels) or without (lower panels) 3 compound treatment. Boxed areas in the left panels are enlarged in the right panels. Scale bars, 200 µm (d) Number of NANOG+ colonies on day 30 under different treatment conditions, without splitting. (e) Number of NANOG+ colonies on day 30 from 3 compound-treated cultures trypsinized as indicated. (f) RT-PCR on iPSC colonies obtained with 3 compound treatment showing reactivated expression of endogenous pluripotency markers. HDF: Human Dermal Fibroblast.
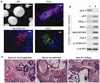
Figure 3. In vitro and in vivo differentiation of iPSCs generated with 3 compound treatment
(a) Micrographs show embryoid bodies (EB) generated from iPSCs and in vitro differentiation into ectodermal (βIII tubulin), mesodermal (brachyury) and endodermal (PDX1) cell types. Scale bars, EB: 100 µm; others 10 µm (b) RT-PCR showing expression of representative lineage markers and the absence of OCT4 mRNA expression in differentiating cells. U- undifferentiated, D- differentiated. (c) Teratomas generated in nude mice from iPSCs (3 independent colonies tested) consist of tissues from all three germ layers. Left panel: 1- muscle, 2- neural epithelium; middle panel: 1- skin, 2- gut epithelium; right panel: 1- bone, 2- cartilage. Scale bars, 20 µm.
Similar articles
- Improved generation of patient-specific induced pluripotent stem cells using a chemically-defined and matrigel-based approach.
Groß B, Sgodda M, Rasche M, Schambach A, Göhring G, Schlegelberger B, Greber B, Linden T, Reinhardt D, Cantz T, Klusmann JH. Groß B, et al. Curr Mol Med. 2013 Jun;13(5):765-76. doi: 10.2174/1566524011313050008. Curr Mol Med. 2013. PMID: 23642058 - Generation of induced pluripotent stem cells without genetic defects by small molecules.
Park HS, Hwang I, Choi KA, Jeong H, Lee JY, Hong S. Park HS, et al. Biomaterials. 2015 Jan;39:47-58. doi: 10.1016/j.biomaterials.2014.10.055. Epub 2014 Nov 15. Biomaterials. 2015. PMID: 25477171 - Optimized Approaches for Generation of Integration-free iPSCs from Human Urine-Derived Cells with Small Molecules and Autologous Feeder.
Li D, Wang L, Hou J, Shen Q, Chen Q, Wang X, Du J, Cai X, Shan Y, Zhang T, Zhou T, Shi X, Li Y, Zhang H, Pan G. Li D, et al. Stem Cell Reports. 2016 May 10;6(5):717-728. doi: 10.1016/j.stemcr.2016.04.001. Epub 2016 Apr 28. Stem Cell Reports. 2016. PMID: 27132887 Free PMC article. - Mechanism of Induction: Induced Pluripotent Stem Cells (iPSCs).
Singh VK, Kumar N, Kalsan M, Saini A, Chandra R. Singh VK, et al. J Stem Cells. 2015;10(1):43-62. J Stem Cells. 2015. PMID: 26665937 Review. - Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells.
Hsu YC, Wu YT, Tsai CL, Wei YH. Hsu YC, et al. Exp Biol Med (Maywood). 2018 Mar;243(6):563-575. doi: 10.1177/1535370218759636. Exp Biol Med (Maywood). 2018. PMID: 29557214 Free PMC article. Review.
Cited by
- Induced pluripotency of human prostatic epithelial cells.
Zhao H, Sun N, Young SR, Nolley R, Santos J, Wu JC, Peehl DM. Zhao H, et al. PLoS One. 2013 May 22;8(5):e64503. doi: 10.1371/journal.pone.0064503. Print 2013. PLoS One. 2013. PMID: 23717621 Free PMC article. - Development of a High-Efficacy Reprogramming Method for Generating Human Induced Pluripotent Stem (iPS) Cells from Pathologic and Senescent Somatic Cells.
Tanaka N, Kato H, Tsuda H, Sato Y, Muramatsu T, Iguchi A, Nakajima H, Yoshitake A, Senbonmatsu T. Tanaka N, et al. Int J Mol Sci. 2020 Sep 15;21(18):6764. doi: 10.3390/ijms21186764. Int J Mol Sci. 2020. PMID: 32942642 Free PMC article. - Chromosome microduplication in somatic cells decreases the genetic stability of human reprogrammed somatic cells and results in pluripotent stem cells.
Yu Y, Chang L, Zhao H, Li R, Fan Y, Qiao J. Yu Y, et al. Sci Rep. 2015 May 12;5:10114. doi: 10.1038/srep10114. Sci Rep. 2015. PMID: 25965553 Free PMC article. - Small-Molecule-Based Lineage Reprogramming Creates Functional Astrocytes.
Tian E, Sun G, Sun G, Chao J, Ye P, Warden C, Riggs AD, Shi Y. Tian E, et al. Cell Rep. 2016 Jul 19;16(3):781-92. doi: 10.1016/j.celrep.2016.06.042. Epub 2016 Jul 7. Cell Rep. 2016. PMID: 27396343 Free PMC article. - An Insight into DNA-free Reprogramming Approaches to Generate Integration-free Induced Pluripotent Stem Cells for Prospective Biomedical Applications.
Borgohain MP, Haridhasapavalan KK, Dey C, Adhikari P, Thummer RP. Borgohain MP, et al. Stem Cell Rev Rep. 2019 Apr;15(2):286-313. doi: 10.1007/s12015-018-9861-6. Stem Cell Rev Rep. 2019. PMID: 30417242 Review.
References
- Takahashi K, et al. Cell. 2007;131:861–872. - PubMed
- Yu J, et al. Science. 2007;318:1917–1920. - PubMed
- Feng B, Ng JH, Heng JCD, Ng HH. Cell Stem Cell. 2009;4:301–312. - PubMed
- Hay ED. Acta Anat. (Basel) 1995;154:8–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources