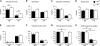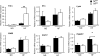Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance - PubMed (original) (raw)
Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance
Guadalupe Sabio et al. Mol Cell Biol. 2010 Jan.
Abstract
Obesity caused by feeding of a high-fat diet (HFD) is associated with an increased activation of c-Jun NH(2)-terminal kinase 1 (JNK1). Activated JNK1 is implicated in the mechanism of obesity-induced insulin resistance and the development of metabolic syndrome and type 2 diabetes. Significantly, Jnk1(-)(/)(-) mice are protected against HFD-induced obesity and insulin resistance. Here we show that an ablation of the Jnk1 gene in skeletal muscle does not influence HFD-induced obesity. However, muscle-specific JNK1-deficient (M(KO)) mice exhibit improved insulin sensitivity compared with control wild-type (M(WT)) mice. Thus, insulin-stimulated AKT activation is suppressed in muscle, liver, and adipose tissue of HFD-fed M(WT) mice but is suppressed only in the liver and adipose tissue of M(KO) mice. These data demonstrate that JNK1 in muscle contributes to peripheral insulin resistance in response to diet-induced obesity.
Figures
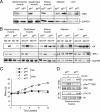
FIG. 1.
Creation of mice with muscle-specific JNK1 deficiency. (A) Extracts prepared from epididymal fat (white adipose tissue), liver, and muscle (gastrocnemius, quadriceps, and soleus) of Mck-Cre+ Jnk1+/+ (MWT) mice and Mck-Cre+ Jnk1LoxP/LoxP (MKO) mice were examined by immunoblot analysis by probing with antibodies to JNK1 and GAPDH. (B) MWT and MKO mice were fed a chow diet (ND) or HFD (HF) for 16 weeks and then fasted overnight. JNK protein activity in epididymal fat (adipose tissue), liver, and muscle was measured in a protein kinase (KA) assay using c-Jun and [γ-32P]ATP as substrates. The cell extracts used for the protein kinase assay were also examined by immunoblot analysis by probing with antibodies to JNK1 and GAPDH. (C) Male MWT and MKO mice (8 to 10 weeks old) were fed either a chow diet (ND) or HFD for 16 weeks. The body weight of the mice was measured (means ± standard deviations [SD]) (n = 10). No significant difference between the body weights of MWT and MKO mice was detected (P > 0.05). (D) Chow-fed MWT and MKO mice were fasted overnight and then treated by intraperitoneal injection of 1.5 mU/kg insulin. Extracts prepared from gastrocnemius muscle at 10 min postinjection were examined by immunoblot analysis using antibodies to the insulin receptor (IR), IRS-1, phospho-Tyr, and IRS-1-Ser307. IP, immunoprecipitation.
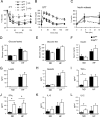
FIG. 2.
Effect of muscle-specific JNK1 deficiency on diet-induced obesity. (A) ITTs of MWT and MKO male mice fed either a chow diet (ND) or an HFD for 16 weeks were performed by intraperitoneal injection of insulin (1.5 U/kg body mass). The concentration of blood glucose was measured (means ± SD) (n = 10). Statistically significant differences between MKO and MWT are indicated (*, P < 0.05). (B) Glucose tolerance tests (GTT) with chow-fed (ND) and HFD-fed MWT and MKO mice were performed by measurement of blood glucose concentrations in animals following intraperitoneal injection of glucose (1 g/kg). The data presented represent the means ± SD (_n_ = 10 to ∼15). No statistically significant differences between MWT and MKO mice were detected (_P_ > 0.05). (C) Glucose-induced insulin release. The effect of the administration of glucose (2 g/kg body mass) by intraperitoneal injection on blood insulin concentrations was examined (means ± SD) (n = 13 to ∼15). No statistically significant differences between MWT and MKO mice were detected (P > 0.05). (D and E) Chow-fed and HFD-fed (HF) MWT and MKO mice were fasted overnight (D) or fed ad libitum (E), and the blood glucose concentration was measured (mean ± SD) (n = 10 to ∼15). No statistically significant differences were detected (P > 0.05). (F to L). The concentrations of insulin, adipokines, and cytokines in blood of chow-fed (ND) and HFD-fed (HF) MWT and MKO mice fasted overnight were measured by enzyme-linked immunosorbent assay (means ± SD) (n = 10 to ∼15). Statistically significant differences are indicated (*, P < 0.05).
FIG. 3.
Effect of muscle-specific deficiency of JNK1 on insulin sensitivity. (A to F) Insulin sensitivity was measured using a hyperinsulinemic-euglycemic clamp with conscious chow-fed (ND) and HFD-fed (HF) MKO and MWT mice. (A) Insulin-stimulated whole-body glucose turnover. (B) Whole-body glycolysis. (C) Whole-body glycogen plus lipid synthesis. (D) Basal HGP. (E) Insulin-stimulated rate of HGP during the clamp assay. (F) Hepatic insulin action expressed as the insulin-mediated percent suppression of basal HGP. The data presented are the means ± standard errors for approximately six to nine experiments. Statistically significant differences between MKO mice and MWT mice are indicated (*, P < 0.05). (G and H) Glucose uptake in white adipose tissue (G) and gastrocnemius muscle (H) was measured in the hyperinsulinemic-euglycemic clamp study. The data are expressed as the percent suppression of glucose uptake caused by feeding of an HFD and presented as the means ± standard errors for approximately four to nine experiments. Statistically significant differences between MKO mice and MWT mice are indicated (*, P < 0.05).
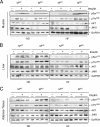
FIG. 4.
Effect of muscle-specific JNK1 deficiency on insulin-stimulated AKT activation. Chow-fed (ND) and HFD-fed (HF) MWT and MKO mice were fasted overnight and treated by intraperitoneal injection of insulin (1.5 U/kg body mass). Extracts prepared from gastrocnemius muscle (A), liver (B), and epididymal adipose tissue (C) at 10 min postinjection were examined by immunoblot analysis with antibodies to JNK1, AKT, phospho-AKT, and GAPDH.
FIG. 5.
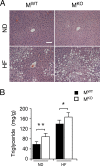
Muscle-specific JNK1 deficiency causes increased diet-induced hepatic steatosis. (A) Chow-fed (ND) and HFD-fed (HF) MWT and MKO mice were fasted overnight. Representative sections of the liver stained with hematoxylin and eosin are presented. Scale bar, 100 μm. (B) The amount of hepatic triglyceride was measured in mice fasted overnight (means ± SD) (n = 10). Statistically significant differences between MKO and MWT are indicated (*, P < 0.05; **, P < 0.01).
FIG. 6.
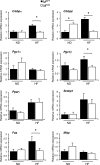
Effect of muscle-specific JNK1 deficiency on hepatic lipogenic gene expression. Gene expression in the liver of chow-fed (ND) and HFD-fed (HF) MWT and MKO mice was measured by quantitative RT-PCR analysis of mRNA. Data for the expression of transcription factors (_C/ebp_α, _C/ebp_β, _Ppar_γ, and Srebp1), coactivators (_Pgc1_α and _Pgc1_β), fatty acid synthase (Fas), and microsomal triglyceride transfer protein (Mttp) mRNA are presented. The relative mRNA expression level was calculated by normalization of the data to the amount of 18S RNA in each sample (means ± SD) (n = 6 to ∼8). Statistically significant differences are indicated (*, P < 0.05).
FIG. 7.
Effect of muscle-specific JNK1 deficiency on hepatic inflammatory gene expression. Gene expression in the liver of chow-fed (ND) and HFD-fed (HF) MWT and MKO mice fasted overnight was measured by quantitative RT-PCR analysis of mRNA (TaqMan assays). Data for the expression of _Tnf_α, Il6, _Ifn_γ, Cd68, Icam1, Lyzs, and cytochrome p450 2E1 (Cye2e1) mRNAs are presented. The relative mRNA expression level was calculated by normalization of the data to the amount of 18S RNA in each sample (means ± SD) (n = 6 to ∼8). Statistically significant differences are indicated (*, P < 0.05; **, P < 0.01).
FIG. 8.
Effect of muscle-specific JNK1 deficiency on blood lipids. (A) HFD-fed MWT and MKO mice were fasted overnight. The amounts of cholesterol, triglyceride, and free fatty acid (FFA) in the blood were measured (means ± SD) (n = 10). Statistically significant differences between MKO and MWT are indicated (*, P < 0.05). (B) Analysis of VLDL, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) in the sera of HFD-fed MWT and MKO mice. Fast-performance liquid chromatography profiles of cholesterol (top) and triglyceride (bottom) are presented (means ± SD) (n = 10).
FIG. 9.
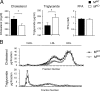
Muscle-specific JNK1 deficiency causes increased diet-induced inflammation of adipose tissue. (A) Chow-fed (ND) and HFD-fed (HF) MWT and MKO mice were fasted overnight. Representative histological sections of epididymal adipose tissue stained with hematoxylin and eosin (left panels) and with an antibody (F4/80) to a macrophage marker (right panels) are presented. Scale bar, 100 μm. (B) Gene expression in epididymal adipose tissue was measured by quantitative RT-PCR analysis of mRNA. The relative mRNA expression level was calculated by normalization of the data to the amount of Gapdh mRNA in each sample (means ± SD) (n = 6 to ∼8). Statistically significant differences are indicated (*, P < 0.05; **, P < 0.01).
Similar articles
- Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance.
Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Singh R, et al. Hepatology. 2009 Jan;49(1):87-96. doi: 10.1002/hep.22578. Hepatology. 2009. PMID: 19053047 Free PMC article. - Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice.
Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS. Zhang X, et al. Diabetes. 2011 Feb;60(2):486-95. doi: 10.2337/db10-0650. Diabetes. 2011. PMID: 21270260 Free PMC article. - JNK1 ablation in mice confers long-term metabolic protection from diet-induced obesity at the cost of moderate skin oxidative damage.
Becattini B, Zani F, Breasson L, Sardi C, D'Agostino VG, Choo MK, Provenzani A, Park JM, Solinas G. Becattini B, et al. FASEB J. 2016 Sep;30(9):3124-32. doi: 10.1096/fj.201600393R. Epub 2016 May 26. FASEB J. 2016. PMID: 27230858 Free PMC article. - A stress signaling pathway in adipose tissue regulates hepatic insulin resistance.
Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. Sabio G, et al. Science. 2008 Dec 5;322(5907):1539-43. doi: 10.1126/science.1160794. Science. 2008. PMID: 19056984 Free PMC article. - cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance.
Sabio G, Davis RJ. Sabio G, et al. Trends Biochem Sci. 2010 Sep;35(9):490-6. doi: 10.1016/j.tibs.2010.04.004. Epub 2010 May 7. Trends Biochem Sci. 2010. PMID: 20452774 Free PMC article. Review.
Cited by
- Selective insulin resistance in adipocytes.
Tan SX, Fisher-Wellman KH, Fazakerley DJ, Ng Y, Pant H, Li J, Meoli CC, Coster AC, Stöckli J, James DE. Tan SX, et al. J Biol Chem. 2015 May 1;290(18):11337-48. doi: 10.1074/jbc.M114.623686. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720492 Free PMC article. - In vivo JNK activation in pancreatic β-cells leads to glucose intolerance caused by insulin resistance in pancreas.
Lanuza-Masdeu J, Arévalo MI, Vila C, Barberà A, Gomis R, Caelles C. Lanuza-Masdeu J, et al. Diabetes. 2013 Jul;62(7):2308-17. doi: 10.2337/db12-1097. Epub 2013 Jan 24. Diabetes. 2013. PMID: 23349497 Free PMC article. - Cow's Milk Bioactive Molecules in the Regulation of Glucose Homeostasis in Human and Animal Studies.
Yuzbashian E, Berg E, de Campos Zani SC, Chan CB. Yuzbashian E, et al. Foods. 2024 Sep 6;13(17):2837. doi: 10.3390/foods13172837. Foods. 2024. PMID: 39272602 Free PMC article. Review. - Novel roles for JNK1 in metabolism.
Belgardt BF, Mauer J, Brüning JC. Belgardt BF, et al. Aging (Albany NY). 2010 Sep;2(9):621-6. doi: 10.18632/aging.100192. Aging (Albany NY). 2010. PMID: 20834068 Free PMC article. - NOD1: An Interface Between Innate Immunity and Insulin Resistance.
Rivers SL, Klip A, Giacca A. Rivers SL, et al. Endocrinology. 2019 May 1;160(5):1021-1030. doi: 10.1210/en.2018-01061. Endocrinology. 2019. PMID: 30807635 Free PMC article. Review.
References
- Aguirre, V., T. Uchida, L. Yenush, R. Davis, and M. F. White. 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275:9047-9054. - PubMed
- Bennett, B. L., Y. Satoh, and A. J. Lewis. 2003. JNK: a new therapeutic target for diabetes. Curr. Opin. Pharmacol. 3:420-425. - PubMed
- Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2:559-569. - PubMed
- Desvergne, B. 2008. PPARdelta/beta: the lobbyist switching macrophage allegiance in favor of metabolism. Cell Metab. 7:467-469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA065861/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CA65861/CA/NCI NIH HHS/United States
- R01 DK080756/DK/NIDDK NIH HHS/United States
- DK80756/DK/NIDDK NIH HHS/United States
- DK52530/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous