Oligodendrocytes: biology and pathology - PubMed (original) (raw)
Review
Oligodendrocytes: biology and pathology
Monika Bradl et al. Acta Neuropathol. 2010 Jan.
Abstract
Oligodendrocytes are the myelinating cells of the central nervous system (CNS). They are the end product of a cell lineage which has to undergo a complex and precisely timed program of proliferation, migration, differentiation, and myelination to finally produce the insulating sheath of axons. Due to this complex differentiation program, and due to their unique metabolism/physiology, oligodendrocytes count among the most vulnerable cells of the CNS. In this review, we first describe the different steps eventually culminating in the formation of mature oligodendrocytes and myelin sheaths, as they were revealed by studies in rodents. We will then show differences and similarities of human oligodendrocyte development. Finally, we will lay out the different pathways leading to oligodendrocyte and myelin loss in human CNS diseases, and we will reveal the different principles leading to the restoration of myelin sheaths or to a failure to do so.
Figures
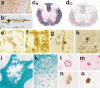
Fig. 1
a–d Detection of oligodendrocytes in paraffin embedded tissue sections. a Mouse cortex, stained by immunocytochemistry for CNPase shows numerous process bearing oligodendrocytes and staining of myelin sheaths, ×120; b high magnification of a CNPase positive oligodendrocyte in the mouse cortex (layer I), showing a small round cell body and few cell processes connected to myelin sheaths, ×1,000; c rat spinal cord stained by in situ hybridization for myelin basic protein mRNA; staining is seen in the cell bodies (e.g., in the gray matter) as well as in oligodendrocyte processes associated with myelin sheaths (dark staining in the white matter), ×50; d rat spinal cord stained by in situ hybridization for proteolipid protein (PLP); the mRNA for PLP is only located within the perinuclear cytoplasm, there is no staining of myelin, ×50; e–h oligodendrocyte pathology in transgenic animals overexpressing proteolipid protein; e and f hemizygous animal, which shows normal myelination, stained by immunocytochemistry for PLP; in the normal animal PLP protein is rarely detected in the cytoplasm of oligodendrocytes; in hemizygous PLP transgenic animals a variable extent of PLP expression is seen in the cytoplasm of oligodendrocytes (e), and this is associated with aberrant formation of myelin-like structures within and adjacent to the cells (f); g–h homozygous animal, which shows extensive dys-myelination; only few oligodendrocytes are preserved, which are connected to myelin sheaths and contain abundant PLP immunoreactivity in their cytoplasm (g); some of the PLP reactive oligodendrocytes show nuclear condensation and fragmentation, consistent with apoptosis (h), ×1,200; i–o neuropathology of progressive multifocal leukoencephalopathy; i multiple small confluent demyelinating lesions in the white and gray matter, giving the impression of a moth eaten pattern of demyelination (×2); k edge of an active demyelinating lesion with numerous macrophages, containing recent (luxol fast blue positive) degradation products; l and m pathologically altered nuclei in PML showing giant nuclei of astrocytes (l) and small oligodendrocyte nuclei with intranuclear inclusion (m); n and o similar nuclei, as shown in l and m contain virus antigen as revealed by immunocytochemistry; ×1,200
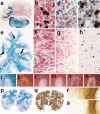
Fig. 2
Myelin and oligodendrocyte pathology in autoimmune encephalomyelitis, multiple sclerosis, and stroke. a–d Chronic autoimmune encephalomyelitis, induced in DA rat by active sensitization with MOG fusion protein; a massive demyelination is seen in the cerebellar white matter, ×6; b–d oligodendrocytes in different lesion stages of EAE; in the peri-plaque white matter myelin (red) is present and multiple oligodendrocytes with PLP mRNA (black) are seen (b); in the active lesions myelin falls apart, myelin fragments are taken up by macrophages (red granules) and oligodendrocytes are lost (c); in more advanced lesions no macrophages with early myelin degradation products are present; numerous oligodendrocytes re-appear in the lesions, apparently recruited from progenitor cells (black cells), followed by rapid and extensive remyelination; immunocytochemistry for PLP and in situ hybridization for PLP mRNA, ×1000; e–h chronic multiple sclerosis case with extensive remyelination within the CNS; This hemispheric brain section contains 3 active lesions, 4 demyelinated plaques, and 8 remyelinated shadow plaques, ×1.2; f–h double staining for PLP protein (red) and PLP mRNA (black) in one of the active lesions shows a similar pattern as described before in EAE; many oligodendrocytes in the peri-plaque white matter (f); oligodendrocyte loss in the zone of active demyelination (g) and reappearance of oligodendrocytes in the inactive zone, closely adjacent to the zone of activity (h), ×500; i–o myelin changes in the initial stage of a lesion in white matter stroke; LFB shows pale myelin staining (i); the axons, stained with Bielschowsky silver impregnation are largely preserved (k); MAG (l) and CNPase (m) are completely lost from the lesions, while the myelin proteins within the compact sheath (PLP; n) or on the oligodendrocyte surface (MOG; o) are preserved, ×20; p–s acute multiple sclerosis with lesions following a pattern of hypoxia-like tissue injury (Pattern III, [109]). p The section contains areas of initial demyelination (i), early active demyelination (a) and late active or inactive portions (d); q serial section of p, stained by immunocytochemistry for PLP; Only the late active and inactive lesions show loss of PLP; in the active portions (a) a minor loss of PLP reactivity is seen, while in the initial lesions PLP reactivity is the same as in the normal appearing white matter, ×3; r and s edge of an active lesion showing partial preservation of immunoreactivity for MOG (r), but extensive and complete loss of MAG(s), ×20
Similar articles
- The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.
Keirstead HS, Blakemore WF. Keirstead HS, et al. Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review. - Oligodendrocyte Development in the Absence of Their Target Axons In Vivo.
Almeida R, Lyons D. Almeida R, et al. PLoS One. 2016 Oct 7;11(10):e0164432. doi: 10.1371/journal.pone.0164432. eCollection 2016. PLoS One. 2016. PMID: 27716830 Free PMC article. - Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis.
Hardy RJ, Friedrich VL Jr. Hardy RJ, et al. Dev Neurosci. 1996;18(4):243-54. doi: 10.1159/000111414. Dev Neurosci. 1996. PMID: 8911764 - Transcriptional and post-transcriptional control of CNS myelination.
Emery B. Emery B. Curr Opin Neurobiol. 2010 Oct;20(5):601-7. doi: 10.1016/j.conb.2010.05.005. Epub 2010 Jun 16. Curr Opin Neurobiol. 2010. PMID: 20558055 Review. - Loss of Tuberous Sclerosis Complex1 in Adult Oligodendrocyte Progenitor Cells Enhances Axon Remyelination and Increases Myelin Thickness after a Focal Demyelination.
McLane LE, Bourne JN, Evangelou AV, Khandker L, Macklin WB, Wood TL. McLane LE, et al. J Neurosci. 2017 Aug 2;37(31):7534-7546. doi: 10.1523/JNEUROSCI.3454-16.2017. Epub 2017 Jul 10. J Neurosci. 2017. PMID: 28694334 Free PMC article.
Cited by
- The Role of Inflammatory Cascade and Reactive Astrogliosis in Glial Scar Formation Post-spinal Cord Injury.
Bhatt M, Sharma M, Das B. Bhatt M, et al. Cell Mol Neurobiol. 2024 Nov 23;44(1):78. doi: 10.1007/s10571-024-01519-9. Cell Mol Neurobiol. 2024. PMID: 39579235 Free PMC article. Review. - Zebrafish as a Model for Multiple Sclerosis.
Maktabi B, Collins A, Safee R, Bouyer J, Wisner AS, Williams FE, Schiefer IT. Maktabi B, et al. Biomedicines. 2024 Oct 16;12(10):2354. doi: 10.3390/biomedicines12102354. Biomedicines. 2024. PMID: 39457666 Free PMC article. Review. - Glial Cells as Key Regulators in Neuroinflammatory Mechanisms Associated with Multiple Sclerosis.
Theophanous S, Sargiannidou I, Kleopa KA. Theophanous S, et al. Int J Mol Sci. 2024 Sep 4;25(17):9588. doi: 10.3390/ijms25179588. Int J Mol Sci. 2024. PMID: 39273535 Free PMC article. Review. - Zuranolone therapy protects frontal cortex neurodevelopment and improves behavioral outcomes after preterm birth.
Moloney RA, Palliser HK, Pavy CL, Shaw JC, Hirst JJ. Moloney RA, et al. Brain Behav. 2024 Sep;14(9):e70009. doi: 10.1002/brb3.70009. Brain Behav. 2024. PMID: 39236116 Free PMC article. - Mechanisms of Transsynaptic Degeneration in the Aging Brain.
Wall RV, Basavarajappa D, Klistoner A, Graham S, You Y. Wall RV, et al. Aging Dis. 2024 Oct 1;15(5):2149-2167. doi: 10.14336/AD.2024.03019. Aging Dis. 2024. PMID: 39191395 Free PMC article. Review.
References
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. - PubMed
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources