Powering through ribosome assembly - PubMed (original) (raw)
Review
. 2009 Dec;15(12):2083-104.
doi: 10.1261/rna.1792109. Epub 2009 Oct 22.
Affiliations
- PMID: 19850913
- PMCID: PMC2779681
- DOI: 10.1261/rna.1792109
Review
Powering through ribosome assembly
Bethany S Strunk et al. RNA. 2009 Dec.
Abstract
Ribosome assembly is required for cell growth in all organisms. Classic in vitro work in bacteria has led to a detailed understanding of the biophysical, thermodynamic, and structural basis for the ordered and correct assembly of ribosomal proteins on ribosomal RNA. Furthermore, it has enabled reconstitution of active subunits from ribosomal RNA and proteins in vitro. Nevertheless, recent work has shown that eukaryotic ribosome assembly requires a large macromolecular machinery in vivo. Many of these assembly factors such as ATPases, GTPases, and kinases hydrolyze nucleotide triphosphates. Because these enzymes are likely regulatory proteins, much work to date has focused on understanding their role in the assembly process. Here, we review these factors, as well as other sources of energy, and their roles in the ribosome assembly process. In addition, we propose roles of energy-releasing enzymes in the assembly process, to explain why energy is used for a process that occurs largely spontaneously in bacteria. Finally, we use literature data to suggest testable models for how these enzymes could be used as targets for regulation of ribosome assembly.
Figures
FIGURE 1.
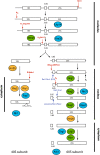
rRNA cleavage involves many steps and energy-consuming factors. (Red arrows) Endonucleolytic steps; (blue arrows) exonucleolytic steps. The steps are labeled with the name of the nuclease if known or suggested, or the name of the cleavage site. Cleavage at site A2 requires prior cleavage at site A1 (Venema and Tollervey 1999; Karbstein 2009), cleavage at site D requires prior cleavage at site A2 (Karbstein 2009), and processing to site B1S requires prior cleavage at site A3 (Karbstein 2009). For simplicity, only the major 60S processing pathway is shown. (Cyan) Putative ATPases (for simplicity, DEAD-box proteins are omitted); (green) GTPases; (orange) kinases.
FIGURE 2.
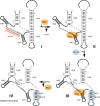
Model for Rix7-catalyzed remodeling of pre-60S subunits. Nsa1 binds to pre-60S subunits containing 27SA rRNA; other early 60S assembly factors including Rrp5, Noc1, and Nop4 are also bound to this intermediate. Rix7 interacts with Nsa1, potentially only in the ubiquitinated/sumoylated form, to remove it from pre-ribosomes (directly or indirectly). Nsa1 removal coincides with loss of the early 60S assembly factors Rrp5, Noc1, and Nop4, and allows binding of the later 60S assembly factors Rsa4, Nop53, Spb1, Nog2, Sda1, Arx1, and Nug1. (Orange) Energy-consuming assembly factors; (yellow) other assembly factors; (blue) Nsa1; (red) Rix7.
FIGURE 3.
Model for a conformational change in the 3′-major domain of 18S rRNA induced by the methylase Nep1. snR57 binds to pre-18S rRNA (complex I) to methylate G1572, marked by an asterisk. This interaction prevents the formation of H33 in mature 18S ribosomes (shown in green in complex IV). Nep1 binds to the GCAACUU sequence upstream of H47 could displace snR57, consistent with suppression of the Nep1 deletion by deletion of snR57. The ensuing formation of H33 (shown in green) allows binding of Rps19 to its suggested site in H45, prior to dissociation of Nep1.
FIGURE 4.
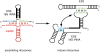
Dissociation of snR30 leads to a conformational switch in pre-ribosomes. Interaction of snR30 (red) with expansion segment 6 (ES6) in 18S rRNA (Fayet-Lebaron et al. 2009) prevents the proposed interaction between ES6 and ES3 (green), as well as a suggested pseudoknot in ES6 (blue) (Alkemar and Nygard 2003, 2006).
FIGURE 5.
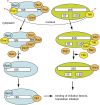
Late cytoplasmic assembly intermediates are regulated in multiple layers. (Blue) 40S subunits; (green) 60S subunits; (orange) energy-consuming assembly factors; (yellow) select other assembly factors. Phosphorylation of Enp1, Rps3, and Tif6, catalyzed by Hrr25, and subsequent dephosphorylation, are required for maturation and export of the 40S and 60S subunits, respectively. Nob1-dependent cleavage at the 3′-end of 18S rRNA is regulated by the kinases Rio1 and Rio2, and the ATPase Fap7. The binding site for the bacterial homolog of Dim1, KsgA, overlaps with the binding site for IF3, indicating that Dim1-containing ribosomes cannot initiate translation. Rli1 binds to both pre-ribosomes and eIF3, indicating a link between assembling and initiating ribosomes. Release of Tif6 requires binding of the Shwachman-Diamond protein Sdo1, perhaps to recruit the GTPase Ria1, which can remove Tif6 from ribosoms in vitro. In addition, Drg1 is required to release Tif6 from pre-ribosomes. Arb1 binds to pre-ribosomes containing Tif6, but also interacts with initiation factors, linking the ribosome assembly and translation machineries.
Similar articles
- Eukaryotic Ribosome Assembly.
Baßler J, Hurt E. Baßler J, et al. Annu Rev Biochem. 2019 Jun 20;88:281-306. doi: 10.1146/annurev-biochem-013118-110817. Epub 2018 Dec 19. Annu Rev Biochem. 2019. PMID: 30566372 Review. - Quantitative proteomic analysis of ribosome assembly and turnover in vivo.
Sykes MT, Shajani Z, Sperling E, Beck AH, Williamson JR. Sykes MT, et al. J Mol Biol. 2010 Oct 29;403(3):331-45. doi: 10.1016/j.jmb.2010.08.005. Epub 2010 Aug 13. J Mol Biol. 2010. PMID: 20709079 Free PMC article. - Atomic structures of the eukaryotic ribosome.
Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N. Klinge S, et al. Trends Biochem Sci. 2012 May;37(5):189-98. doi: 10.1016/j.tibs.2012.02.007. Epub 2012 Mar 20. Trends Biochem Sci. 2012. PMID: 22436288 Review. - [Ribosome: lessons of a molecular factory construction].
Sergeeva OV, Sergiev PV, Bogdanov AA, Dontsova OA. Sergeeva OV, et al. Mol Biol (Mosk). 2014 Jul-Aug;48(4):543-60. Mol Biol (Mosk). 2014. PMID: 25842841 Review. Russian. - Reconstitution of 30S ribosomal subunits in vitro using ribosome biogenesis factors.
Tamaru D, Amikura K, Shimizu Y, Nierhaus KH, Ueda T. Tamaru D, et al. RNA. 2018 Nov;24(11):1512-1519. doi: 10.1261/rna.065615.118. Epub 2018 Aug 3. RNA. 2018. PMID: 30076205 Free PMC article.
Cited by
- One core, two shells: bacterial and eukaryotic ribosomes.
Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M. Melnikov S, et al. Nat Struct Mol Biol. 2012 Jun 5;19(6):560-7. doi: 10.1038/nsmb.2313. Nat Struct Mol Biol. 2012. PMID: 22664983 Review. - Chaperoning ribosome assembly.
Karbstein K. Karbstein K. J Cell Biol. 2010 Apr 5;189(1):11-2. doi: 10.1083/jcb.201002102. J Cell Biol. 2010. PMID: 20368615 Free PMC article. - Maturation of eukaryotic ribosomes: acquisition of functionality.
Panse VG, Johnson AW. Panse VG, et al. Trends Biochem Sci. 2010 May;35(5):260-6. doi: 10.1016/j.tibs.2010.01.001. Trends Biochem Sci. 2010. PMID: 20137954 Free PMC article. Review. - Strong dependence between functional domains in a dual-function snoRNA infers coupling of rRNA processing and modification events.
Liang XH, Liu Q, Liu Q, King TH, Fournier MJ. Liang XH, et al. Nucleic Acids Res. 2010 Jun;38(10):3376-87. doi: 10.1093/nar/gkq043. Epub 2010 Feb 9. Nucleic Acids Res. 2010. PMID: 20144950 Free PMC article. - MTG1 couples mitoribosome large subunit assembly with intersubunit bridge formation.
Kim HJ, Barrientos A. Kim HJ, et al. Nucleic Acids Res. 2018 Sep 19;46(16):8435-8453. doi: 10.1093/nar/gky672. Nucleic Acids Res. 2018. PMID: 30085276 Free PMC article.
References
- Algire MA, Maag D, Lorsch JR. π Release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell. 2005;20:251–262. - PubMed
- Alkemar G, Nygard O. Probing the secondary structure of expansion segment ES6 in 18S ribosomal RNA. Biochemistry. 2006;45:8067–8078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources