CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury - PubMed (original) (raw)
CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury
Vivek D Desai et al. Differentiation. 2010 Feb.
Abstract
Hyaluronan is an oligosaccharide found in the pericellular matrix of numerous cell types and hyaluronan-induced signaling is known to facilitate fibrosis and cancer progression in some tissues. Hyaluronan is also commonly instilled into the eye during cataract surgery to protect the corneal endothelium from damage. Despite this, little is known about the distribution of hyaluronan or its receptors in the normal ocular lens. In this study, hyaluronan was found throughout the mouse lens, with apparently higher concentrations in the lens epithelium. CD44, a major cellular receptor for hyaluronan, is expressed predominately in mouse secondary lens fiber cells born from late embryogenesis into adulthood. Surgical removal of lens fiber cells from adult mice resulted in a robust upregulation of CD44 protein, which preceded the upregulation of alpha-smooth muscle actin expression typically used as a marker of epithelial-mesenchyma transition in this model of lens epithelial cell fibrosis. Mice lacking the CD44 gene had morphologically normal lenses with a response to lens fiber cell removal similar to wildtype, although they exhibited an increase in cell-associated hyaluronan. Overall, these data suggest that lens cells have a hyaluronan-containing pericellular matrix whose structure is partially regulated by CD44. Further, these data indicate that CD44 upregulation in the lens epithelium may be an earlier marker of lens injury responses in the mouse lens than the upregulation of alpha-smooth muscle actin.
2009 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures
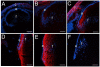
Figure 1
CD44 expression initiates in cortical lens fiber cells at around 16.5 dpc in the mouse lens and is absent from the embryonic lens nucleus A) 14 dpc eye showing a lack of CD44 staining (red) in both the primary and early secondary lens fibers as well as the lens epithelium B) 16.5 dpc lens showing the early onset of CD44 expression in the newly forming secondary fibers. C) newborn lens showing the further upregulation of CD44 protein expression in cortical lens fibers and its absence in the lens epithelium D) 2 weeks postnatal lens showing maintained CD44 expression in cortical lens fibers and its absence in the lens epithelium E) 7 weeks postnatal lens showing maintained CD44 expression in cortical lens fibers and its absence in the lens epithelium F) 7 weeks postnatal CD44 null stained as in panel E demonstrating the specificity of the CD44 antibody used in this study. Abbreviations: c, cornea; e, lens epithelium; f, lens fibers. Blue, nucleic acid stain; Red, CD44. Scale bars A=108 μm; B,C,D,E,F=77 μm
Figure 2
Hyaluronic acid is found in the pericellular matrix of the lens and increases in the absence of CD44. A) 14.5 dpc mouse lens showing hyluronan staining in all lens cells prior to the onset of CD44 expression B) Wildtype adult lens showing hyaluronan staining in both the lens epithelium and fibers. Inset shows a higher magnification of the lens fibers showing staining along the lateral lens fiber cell membrane. C) CD44 null adult lens showing elevated hyaluronan staining in both the lens epithelium and fibers D) CD44 null lens with hyaluronic acid binding protein omitted from the staining reaction as a negative control. Blue, nucleic acid stain; Green, hyaluronic acid. Scale bars all equal 77 μm.
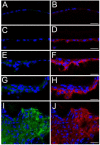
Figure 3
CD44 protein levels upregulate in the lens epithelium prior to α smooth muscle actin after fiber cell removal. A, B- the lens epithelium time zero after fiber cell removal showing a little CD44 (B) or α-smooth muscle actin staining (A); C, D- lens epithelial cells remaining in the eye 12 hours after fiber cell removal showing CD44 staining (D) but no upregulation of α-smooth muscle actin protein expression(C) ; E, F-lens epithelial cells 24 hours after fiber cell removal showing increased levels of CD44 (F) and α-smooth muscle actin protein (E) ; G, H-Lens epithelial cells 48 hours after fiber cell removal showing robust CD44 (H) and α-smooth muscle actin protein (G) expression; I, J lens capsular bag five days after lens fiber cell removal showing a fibrotic mass of cells staining positive for both α-smooth muscle actin (I) and CD44 protein (J) Red, CD44; Blue, nucleic acid stain; Green, α-smooth muscle actin; Scale bars- 24 μm
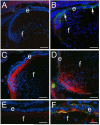
Figure 4
CD44 protein levels increase after the upregulation of α-smooth muscle actin in lens epithelial cells lacking β1-integrin. A) 16.5 dpc wildtype mouse lens showing little CD44 or αSMA staining B) 16.5 dpc β1-integrin null lens showing upregulation of αSMA but little to no D44 staining in the lens epithelium; C) Transition zone of a mewborn wildtype lens showing upregulation of CD44 expression in cortical fibers. D) Transition zone of a newborn β1-integrin null lens showing CD44 in both the cortical fibers and in lens epithelial cells also expressing αSMA. E) high magnification view of the lens epithelium of the wildtype newborn lens shown in C showing a lack of both CD44 and αSMA staining in the lens epithelium. F) high magnification view of the β1-integrin null lens epithelium shown in D showing both CD44 and αSMA staining; Red, CD44; Blue, nucleic acid stain; Green, α-smooth muscle actin; Scale bars-Panels A-D=77 μm, E,F=24 μm
Figure 5
CD44 splicing is not altered following fiber cell removal. rt-PCR analysis of CD44 expression in the whole lens and the lens epithelium after lens fiber extraction using primers that amplify across the major region of CD44 alternate splicing. The canonical form of CD44 which does not utilize the variable exons gives a product of 180 bp (arrow). The differences in CD44 level in the lens epithelium following surgery were then determined by quantitative real time rt-PCR by the ΔΔcT method and are expressed as fold differences in expression level from samples collected immediately after surgery (0 hr). All of the post surgery samples were found to be significantly different than the 0 hr time point with p≤0.02. The conventional rt-PCR was controlled by performing controls lacking reverse transcriptase (-rt) and amplification of the samples with primers for β2-microglobulin (β2MG). M- marker; L- entire adult mouse lens; 12 hr- twelve hours following fiber cell extraction; 24 hr- 24 hours following fiber cell extraction; 48 hr- 48 hours following fiber cell extraction.
Figure 6
CD44 is not essential for α-smooth muscle actin expression in the lens following injury. A) CD44 null lens epithelium immediately following fiber cell removal; B) CD44 null lens epithelium 24 hours after fiber cell removal; C) CD44 null lens epithelium 48 hours after fiber cell removal showing robust αSMA upregulation. Green- α-smooth muscle actin; Blue-nucleic acid stain; Scale bars= 77 μm
Similar articles
- Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction.
Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, Tanihara H, Saya H. Takahashi E, et al. J Biol Chem. 2010 Feb 5;285(6):4060-4073. doi: 10.1074/jbc.M109.056523. Epub 2009 Dec 4. J Biol Chem. 2010. PMID: 19965872 Free PMC article. - Immunolocalization of hyaluronan and CD44 in quiescent and proliferating human lens epithelial cells.
Saika S, Kawashima Y, Miyamoto T, Okada Y, Tanaka S, Yamanaka O, Ohnishi Y, Ooshima A, Yamanaka A. Saika S, et al. J Cataract Refract Surg. 1998 Sep;24(9):1266-70. doi: 10.1016/s0886-3350(98)80025-4. J Cataract Refract Surg. 1998. PMID: 9768406 - CD44 and hyaluronan promote the bone morphogenetic protein 7 signaling response in murine chondrocytes.
Luo N, Knudson W, Askew EB, Veluci R, Knudson CB. Luo N, et al. Arthritis Rheumatol. 2014 Jun;66(6):1547-58. doi: 10.1002/art.38388. Arthritis Rheumatol. 2014. PMID: 24497488 Free PMC article. - Involvement of hyaluronan and CD44 in cancer and viral infections.
Heldin P, Kolliopoulos C, Lin CY, Heldin CH. Heldin P, et al. Cell Signal. 2020 Jan;65:109427. doi: 10.1016/j.cellsig.2019.109427. Epub 2019 Oct 22. Cell Signal. 2020. PMID: 31654718 Review. - The roles of hyaluronan/RHAMM/CD44 and their respective interactions along the insidious pathways of fibrosarcoma progression.
Nikitovic D, Kouvidi K, Karamanos NK, Tzanakakis GN. Nikitovic D, et al. Biomed Res Int. 2013;2013:929531. doi: 10.1155/2013/929531. Epub 2013 Sep 5. Biomed Res Int. 2013. PMID: 24083250 Free PMC article. Review.
Cited by
- The aging mouse lens transcriptome.
Faranda AP, Shihan MH, Wang Y, Duncan MK. Faranda AP, et al. Exp Eye Res. 2021 Aug;209:108663. doi: 10.1016/j.exer.2021.108663. Epub 2021 Jun 11. Exp Eye Res. 2021. PMID: 34119483 Free PMC article. - Aldose reductase inhibition enhances lens regeneration in mice.
Zukin LM, Pedler MG, Chyung K, Seiwald S, Lenhart P, Shieh B, Petrash JM. Zukin LM, et al. Chem Biol Interact. 2019 Jul 1;307:58-62. doi: 10.1016/j.cbi.2019.04.021. Epub 2019 Apr 23. Chem Biol Interact. 2019. PMID: 31026421 Free PMC article. - The effect of sex on the mouse lens transcriptome.
Faranda AP, Shihan MH, Wang Y, Duncan MK. Faranda AP, et al. Exp Eye Res. 2021 Aug;209:108676. doi: 10.1016/j.exer.2021.108676. Epub 2021 Jun 17. Exp Eye Res. 2021. PMID: 34146586 Free PMC article. - Derivation of multiple cranial tissues and isolation of lens epithelium-like cells from human embryonic stem cells.
Mengarelli I, Barberi T. Mengarelli I, et al. Stem Cells Transl Med. 2013 Feb;2(2):94-106. doi: 10.5966/sctm.2012-0100. Epub 2013 Jan 22. Stem Cells Transl Med. 2013. PMID: 23341438 Free PMC article. - Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.
Cvekl A, Camerino MJ. Cvekl A, et al. Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
References
- Banh A, Bantseev V, Choh V, Moran KL, Sivak JG. Prog Retin Eye Res. 2005 - PubMed
- Wride MA. Differentiation. 1996;61:77–93. - PubMed
- Coulombre AJ. Ophthalmology. 1979;86:1559–1570. - PubMed
- Limburg H, von-Bischhoffshausen F. Barria, Gomez P, Silva JC, Foster A. Br J Ophthalmol. 2008;92:315–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY015279/EY/NEI NIH HHS/United States
- P20 RR016472/RR/NCRR NIH HHS/United States
- R01 EY015279/EY/NEI NIH HHS/United States
- R01 EY015279-06/EY/NEI NIH HHS/United States
- P20RR16472/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous