Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) - PubMed (original) (raw)
Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR)
W G Dixon et al. Ann Rheum Dis. 2010 Mar.
Abstract
Background: The risk of tuberculosis (TB) in patients with rheumatoid arthritis (RA) is thought to be increased following anti-tumour necrosis factor (anti-TNF) therapy, with a proposed differential risk between the anti-TNF drugs etanercept (ETA), infliximab (INF) and adalimumab (ADA).
Objective: To compare directly the risk between drugs, to explore time to event, site of infection and the role of ethnicity.
Methods: Data from the British Society for Rheumatology Biologics Register (BSRBR), a national prospective observational study, were used to compare TB rates in 10 712 anti-TNF treated patients (3913 ETA, 3295 INF, 3504 ADA) and 3232 patients with active RA treated with traditional disease-modifying antirheumatic drugs.
Results: To April 2008, 40 cases of TB were reported, all in the anti-TNF cohort. The rate of TB was higher for the monoclonal antibodies ADA (144 events/100,000 person-years) and INF (136/100,000 person-years) than for ETA (39/100,000 person-years). After adjustment, the incidence rate ratio compared with ETA-treated patients was 3.1 (95% CI 1.0 to 9.5) for INF and 4.2 (1.4 to 12.4) for ADA. The median time to event was lowest for INF (5.5 months) compared with ETA (13.4 months) and ADA (18.5 months). 13/40 cases occurred after stopping treatment. 25/40 (62%) cases were extrapulmonary, of which 11 were disseminated. Patients of non-white ethnicity had a sixfold increased risk of TB compared with white patients treated with anti-TNF therapy.
Conclusion: The rate of TB in patients with RA treated with anti-TNF therapy was three- to fourfold higher in patients receiving INF and ADA than in those receiving ETA.
Conflict of interest statement
Competing interests: None.
Figures
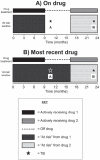
Figure 1
Models for attributing tuberculosis (TB) to drug treatment. (A) “On drug”. Patient-years and adverse events were attributed to each drug only while the patient was actively receiving that drug. Event A was not attributed to any drug, while event B was attributed to drug 2. (B) “Most recent drug”. Patient-years were accrued for each drug from the start date of that drug until the date of switching to the next anti-tumour necrosis factor drug, irrespective of drug discontinuation. Follow-up was censored at the most recently completed consultant follow-up or death, whichever came first, for all models.
Figure 2
Cumulative incidence of tuberculosis (TB) following first exposure to anti-tumour necrosis factor (anti-TNF) therapy (most recent drug model, with person-years censored at death, last returned follow-up form, or date of switching to second anti-TNF). Numbers in table represent the number of patients eligible for follow-up at the specified follow-up time points. ADA, adalimumab; DMARD, disease-modifying antirheumatic drug; ETA, etanercept; INF, infliximab.
Similar articles
- Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register.
Harrison MJ, Dixon WG, Watson KD, King Y, Groves R, Hyrich KL, Symmons DP; British Society for Rheumatology Biologics Register Control Centre Consortium; BSRBR. Harrison MJ, et al. Ann Rheum Dis. 2009 Feb;68(2):209-15. doi: 10.1136/ard.2007.087288. Epub 2008 Apr 2. Ann Rheum Dis. 2009. PMID: 18385277 Free PMC article. - Venous thrombotic events are not increased in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register.
Davies R, Galloway JB, Watson KD, Lunt M, Symmons DP, Hyrich KL; BSRBR Control Centre Consortium, British Society for Rheumatology Biologics Register. Davies R, et al. Ann Rheum Dis. 2011 Oct;70(10):1831-4. doi: 10.1136/ard.2011.153536. Epub 2011 Jul 22. Ann Rheum Dis. 2011. PMID: 21784722 Free PMC article. - Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.
van Herwaarden N, den Broeder AA, Jacobs W, van der Maas A, Bijlsma JW, van Vollenhoven RF, van den Bemt BJ. van Herwaarden N, et al. Cochrane Database Syst Rev. 2014 Sep 29;(9):CD010455. doi: 10.1002/14651858.CD010455.pub2. Cochrane Database Syst Rev. 2014. PMID: 25264908 Updated. Review. - Adalimumab, etanercept, infliximab, and the risk of tuberculosis: data from clinical trials, national registries, and postmarketing surveillance.
Cantini F, Niccoli L, Goletti D. Cantini F, et al. J Rheumatol Suppl. 2014 May;91:47-55. doi: 10.3899/jrheum.140102. J Rheumatol Suppl. 2014. PMID: 24789000 Review.
Cited by
- Therapeutic potential of Leea asiatica: Chemical isolation and validation of ethnomedicinal claims through in vitro and in silico assessment of antioxidant and anti-inflammatory properties.
Joshi KR, Devkota HP, Al-Mutairi KA, Sugimura K, Yahara S, Khadka R, Thapa S, Shekh MU, Poudel S, Watanabe T. Joshi KR, et al. Heliyon. 2024 Sep 18;10(19):e38074. doi: 10.1016/j.heliyon.2024.e38074. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386820 Free PMC article. - Comparisons of infection events associated with tumor necrosis factor inhibitors in patients with inflammatory arthritis: A systematic review and network meta-analysis.
Jiang Z, Zou Y, Li G, Zhao S, Zhang W. Jiang Z, et al. Front Pharmacol. 2024 Jul 11;15:1376262. doi: 10.3389/fphar.2024.1376262. eCollection 2024. Front Pharmacol. 2024. PMID: 39070789 Free PMC article. - Is yearly interferon gamma release assay latent tuberculosis infection screening warranted among patients with rheumatological diseases on disease-modifying drugs in non-endemic settings?
Palacios CF, Chowdhary V, Hao R, Danve A, Malinis M. Palacios CF, et al. PLoS One. 2024 Jul 3;19(7):e0306337. doi: 10.1371/journal.pone.0306337. eCollection 2024. PLoS One. 2024. PMID: 38959249 Free PMC article. - PERFECTRA: a pragmatic, multicentre, real-life study comparing treat-to-target strategies with baricitinib versus TNF inhibitors in patients with active rheumatoid arthritis after failure on csDMARDs.
van de Laar CJ, Oude Voshaar MAH, Ten Klooster P, Tedjo DI, Bos R, Jansen T, Willemze A, Versteeg GA, Goekoop-Ruiterman YPM, Kroot EJ, van de Laar M. van de Laar CJ, et al. RMD Open. 2024 May 30;10(2):e004291. doi: 10.1136/rmdopen-2024-004291. RMD Open. 2024. PMID: 38816210 Free PMC article. Clinical Trial.
References
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9 - PubMed
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. [see comment]. N Engl J Med 2001;345:1098–104 - PubMed
- Mohan AK, Cote TR, Block JA, et al. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. [see comment]. Clin Infect Dis 2004;39:295–9 - PubMed
- Wallis RS, Broder MS, Wong JY, et al. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. [see comment]. Clin Infect Dis 2004;38:1261–5 - PubMed
- Wallis RS, Broder M, Wong J, et al. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis 2004;39:1254–5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous