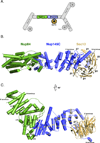Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice - PubMed (original) (raw)
. 2009 Nov;16(11):1173-7.
doi: 10.1038/nsmb.1713. Epub 2009 Oct 25.
Affiliations
- PMID: 19855394
- PMCID: PMC3398507
- DOI: 10.1038/nsmb.1713
Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice
Stephen G Brohawn et al. Nat Struct Mol Biol. 2009 Nov.
Abstract
Nuclear pore complexes (NPCs) facilitate all nucleocytoplasmic transport. These massive protein assemblies are modular, with a stable structural scaffold supporting more dynamically attached components. The scaffold is made from multiple copies of the heptameric Y complex and the heteromeric Nic96 complex. We previously showed that members of these core subcomplexes specifically share an ACE1 fold with Sec31 of the COPII vesicle coat, and we proposed a lattice model for the NPC based on this commonality. Here we present the crystal structure of the heterotrimeric 134-kDa complex of Nup84-Nup145C-Sec13 of the Y complex. The heterotypic ACE1 interaction of Nup84 and Nup145C is analogous to the homotypic ACE1 interaction of Sec31 that forms COPII lattice edge elements and is inconsistent with the alternative 'fence-like' NPC model. We construct a molecular model of the Y complex and compare the architectural principles of COPII and NPC lattices.
Figures
Figure 1. Structure of Nup84•Nup145C•Sec13
(A) Schematic diagram of the Y-complex of the nuclear pore complex (NPC). The crystallized trimeric segment is colored. (B-D) The overall structure of the heterotrimeric Nup84•Nup145C•Sec13 complex is shown with Nup84 in green, Nup145C in blue, and Sec13 in light orange. (B) The β-propeller composed of blades 1–6 from Sec13 and blade 7 from Nup145C is labeled. (C) Structure rotated by 90°, with secondary structure elements of Nup84 and Nup145C labeled.
Figure 2. The crown•crown interaction of Nup84•Nup145C is analogous to Sec31•Sec31
The ancestral coatomer element 1 (ACE1) crown•crown interaction between Nup145C and Nup84 is shown in (A) and between two molecules of Sec31 in (B). In (B), The Sec31 interaction is shown non-domain swapped and the remainders of the molecules are removed for clarity (see main text). Analogous juxtaposition of crown helices α6–α8 is observed in both structures.
Figure 3. Comparison of edge elements in the NPC and COPII lattices
The lattice edge element Nup84•Nup145C in the NPC and Sec31•Sec31 (PDB: 2PM6) in the COPII vesicle coat are shown as cartoons in a half-transparent surface. The two ACE1 units in each edge element are colored by module, with trunks orange and crowns blue. A yellow line indicates the interface between crown modules. The structures are shown from a top view in (A) (180° rotated from Figure 1A) and a side view rotated by 90° in (B). The analogous crown•crown interactions result in edge elements that share a common architectural arrangement. Viewed from the top, the Nup84•Nup145C edge element is bent ~10° from horizontal, while the Sec31•Sec31 edge is essentially straight. Viewed from the side, the crystal structures of the edge elements show dramatically different angles with the Nup84•Nup145C edge 45° more acute than Sec31•Sec31. The angle observed in the Nup84•Nup145C edge corresponds closely to the angle the Sec31•Sec31 interface was modeled to for fitting into the EM reconstructions of the COPII cage and coat ,. The proposed position of the nuclear envelope (NE) membrane relative to the NPC edge element shown in (B) is analogous to the known position of the COPII vesicle membrane relative to the COPII edge element.
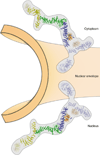
Figure 4. Nup84•Nup145C is a membrane curvature-stabilizing edge element in the NPC lattice
A composite atomic model for the Y-complex of the NPC emphasizing the role of the Nup84•Nup145C edge element as a membrane curvature-stabilizing unit, analogous to the Sec31•Sec31 edge element in COPII vesicle coats. The long arm of the Y-complex is a composite model from crystal structures and is shown with Nup145C blue, Sec13 orange, Nup84 green, and Nup133 yellow. The relative position of the N-terminal propeller of Nup133 (yellow) and the short arm components Nup120 (blue) and Nup85 (blue)•Seh1 (orange) are more tentatively placed and shown half-transparent (see text for details). The long axis of the Y-complex is oriented along the positively curved NE membrane with the concave face of the Nup84•Nup145C edge element facing the lipid bilayer. This orientation is analogous to that of the Sec31•Sec31 edge element in the COPII coat and is consistent with the evolutionary relationship between the NPC and COPII vesicle coat lattices. Importantly, while the Y-complex is shown facing the membrane, it is not predicted to directly contact the NE. Rather, other Nups are predicted to play roles that correspond to adaptor complexes in other vesicle coating systems that link the membrane curvature-stabilizing coat (the Y-complex) to the NE.
Similar articles
- Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat.
Whittle JR, Schwartz TU. Whittle JR, et al. J Cell Biol. 2010 Aug 9;190(3):347-61. doi: 10.1083/jcb.201003092. J Cell Biol. 2010. PMID: 20696705 Free PMC article. - Structural evidence for common ancestry of the nuclear pore complex and vesicle coats.
Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Brohawn SG, et al. Science. 2008 Nov 28;322(5906):1369-73. doi: 10.1126/science.1165886. Epub 2008 Oct 30. Science. 2008. PMID: 18974315 Free PMC article. - Structure of a trimeric nucleoporin complex reveals alternate oligomerization states.
Nagy V, Hsia KC, Debler EW, Kampmann M, Davenport AM, Blobel G, Hoelz A. Nagy V, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17693-8. doi: 10.1073/pnas.0909373106. Epub 2009 Oct 1. Proc Natl Acad Sci U S A. 2009. PMID: 19805193 Free PMC article. - Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges.
Leksa NC, Schwartz TU. Leksa NC, et al. Nucleus. 2010 Jul-Aug;1(4):314-8. doi: 10.4161/nucl.1.4.11798. Epub 2010 Mar 12. Nucleus. 2010. PMID: 21327078 Free PMC article. Review. - Toward the atomic structure of the nuclear pore complex: when top down meets bottom up.
Hoelz A, Glavy JS, Beck M. Hoelz A, et al. Nat Struct Mol Biol. 2016 Jul;23(7):624-30. doi: 10.1038/nsmb.3244. Epub 2016 Jun 6. Nat Struct Mol Biol. 2016. PMID: 27273515 Free PMC article. Review.
Cited by
- Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines.
Ori A, Banterle N, Iskar M, Andrés-Pons A, Escher C, Khanh Bui H, Sparks L, Solis-Mezarino V, Rinner O, Bork P, Lemke EA, Beck M. Ori A, et al. Mol Syst Biol. 2013;9:648. doi: 10.1038/msb.2013.4. Mol Syst Biol. 2013. PMID: 23511206 Free PMC article. - ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p.
Copic A, Latham CF, Horlbeck MA, D'Arcangelo JG, Miller EA. Copic A, et al. Science. 2012 Mar 16;335(6074):1359-62. doi: 10.1126/science.1215909. Epub 2012 Feb 2. Science. 2012. PMID: 22300850 Free PMC article. - Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat.
Whittle JR, Schwartz TU. Whittle JR, et al. J Cell Biol. 2010 Aug 9;190(3):347-61. doi: 10.1083/jcb.201003092. J Cell Biol. 2010. PMID: 20696705 Free PMC article. - Co-translational assembly orchestrates competing biogenesis pathways.
Seidel M, Becker A, Pereira F, Landry JJM, de Azevedo NTD, Fusco CM, Kaindl E, Romanov N, Baumbach J, Langer JD, Schuman EM, Patil KR, Hummer G, Benes V, Beck M. Seidel M, et al. Nat Commun. 2022 Mar 9;13(1):1224. doi: 10.1038/s41467-022-28878-5. Nat Commun. 2022. PMID: 35264577 Free PMC article. - Dynamics of the plant nuclear envelope and nuclear pore.
Boruc J, Zhou X, Meier I. Boruc J, et al. Plant Physiol. 2012 Jan;158(1):78-86. doi: 10.1104/pp.111.185256. Epub 2011 Sep 26. Plant Physiol. 2012. PMID: 21949214 Free PMC article. Review. No abstract available.
References
- Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. - PubMed
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. - PubMed
- Elad N, Maimon T, Frenkiel-Krispin D, Lim RY, Medalia O. Structural analysis of the nuclear pore complex by integrated approaches. Curr Opin Struct Biol. 2009;19:226–232. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases