Revised role of glycosaminoglycans in TAT protein transduction domain-mediated cellular transduction - PubMed (original) (raw)
Revised role of glycosaminoglycans in TAT protein transduction domain-mediated cellular transduction
Jacob M Gump et al. J Biol Chem. 2010.
Abstract
Cellular uptake of the human immunodeficiency virus TAT protein transduction domain (PTD), or cell-penetrating peptide, has previously been surmised to occur in a manner dependent on the presence of heparan sulfate proteoglycans that are expressed ubiquitously on the cell surface. These acidic polysaccharides form a large pool of negative charge on the cell surface that TAT PTD binds avidly. Additionally, sulfated glycans have been proposed to aid in the interaction of TAT PTD and other arginine-rich PTDs with the cell membrane, perhaps aiding their translocation across the membrane. Surprisingly, however, TAT PTD-mediated induction of macropinocytosis and cellular transduction occurs in the absence of heparan sulfate and sialic acid. Using labeled TAT PTD peptides and fusion proteins, in addition to TAT PTD-Cre recombination-based phenotypic assays, we show that transduction occurs efficiently in mutant Chinese hamster ovary cell lines deficient in glycosaminoglycans and sialic acids. Similar results were obtained in cells where glycans were enzymatically removed. In contrast, enzymatic removal of proteins from the cell surface completely ablated TAT PTD-mediated transduction. Our findings support the hypothesis that acidic glycans form a pool of charge that TAT PTD binds on the cell surface, but this binding is independent of the PTD-mediated transduction mechanism and the induction of macropinocytotic uptake by TAT PTD.
Figures
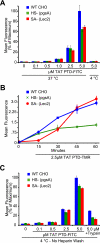
FIGURE 1.
TAT PTD peptide cell association and cell-surface binding in glycan-deficient cells. A, TAT PTD peptide cell association in parental CHO-K1 and derivative glycan mutant pgsA and Lec2 cell lines. Cells were treated with the indicated concentrations of fluoresceinated TAT PTD peptide (TAT PTD-FITC) at 37 °C for 1 h, followed by washes with PBS, heparin, and trypsin to remove extracellular peptide. Cells were placed on ice and immediately analyzed by flow cytometry. B, time course of TAT PTD cell association in parental and glycan mutant cell lines. Cells were treated with 2.5 μ
m
TAT PTD-TMR as described for A for the indicated times. C, TAT PTD peptide cell-surface binding at 4 °C. Cells were treated for 15 min with TAT PTD-FITC peptide, followed by washes with PBS, nonenzymatic cell dissociation, and flow cytometry. WT, wild-type.
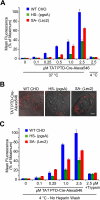
FIGURE 2.
TAT PTD-Cre fusion protein cell association and cell-surface binding in glycan-deficient cells. A, flow cytometric analysis of cells treated for 1 h at 37 °C at the indicated concentrations of recombinant TAT PTD-Cre labeled with Alexa Fluor 546. Cells were washed thoroughly with PBS, heparin, and trypsin to remove extracellular TAT fusion protein. B, live cell photomicrographs of the indicated cell lines treated with 1 μ
m
TAT PTD-Cre-Alexa Fluor 546 for 1 h at 37 °C. TAT PTD-Cre treatment was followed by washes with PBS and heparin. Scale bar = 5 μm. C, TAT PTD-Cre-Alexa Fluor 546 cell-surface binding at 4 °C. Cells were treated for 15 min with TAT PTD-Cre-Alexa Fluor 546, followed by PBS wash, nonenzymatic cell dissociation, and flow cytometry. WT, wild-type.
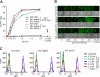
FIGURE 3.
TAT PTD-Cre transduction occurs in the absence of glycosaminoglycans and SAs in parental CHO and glycan-deficient cell lines. A, TAT PTD-Cre recombination in CHO-K1 and derivative glycan mutant pgsA and Lec2 cells with a stably integrated Lox-STOP-Lox-GFP expression construct. Cells were treated with purified recombinant TAT PTD-Cre fusion protein or control recombinant Cre (no TAT PTD) at the indicated concentrations for 1 h, followed by trypsinization and replating. Flow cytometry was performed at 24 h after Cre addition. As indicated, control cells were treated with cytochalasin D to inhibit macropinocytosis for 1 h at 37 °C immediately preceding and during TAT PTD-Cre treatment. B, photomicrographs of TAT PTD-Cre recombination-induced GFP expression in parental and glycan mutant cells with stably integrated LSL-GFP construct. C, FACS profiles of representative samples from the experiment in A. WT, wild-type.
FIGURE 4.
Macropinocytotic inhibitors and TAT PTD-Cre transduction in glycan-deficient cells. A, CHO-K1, pgsA, and Lec2 cells with a stably integrated LSL-GFP construct treated with the indicated concentrations of amiloride, an inhibitor of macropinocytosis, for 1 h. Cells were then treated with TAT PTD-Cre protein in the presence of amiloride for 1 h, followed by trypsinization and replating. GFP expression was assayed by flow cytometry at 24 h after TAT PTD-Cre addition. Inset, cell viability as assayed by flow cytometry immediately following TAT PTD-Cre treatment. B, treatment with wortmannin, a kinase inhibitor, as described for A. C, treatment with cytochalasin D, an inhibitor of F-actin and macropinocytosis, as described for A. D, depletion of cell-surface proteins by trypsin. CHO-K1 LSL cells were treated with trypsin or cell dissociation solution (CDS) for 30 min, followed by washes with PBS and trypsin inhibitor and treatment with TAT PTD-Cre for 1 h. Cells were then washed, trypsinized, and replated. GFP expression was assayed by FACS at 24 h following TAT PTD-Cre treatment. WT, wild-type.
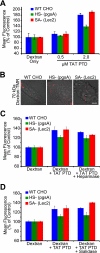
FIGURE 5.
TAT PTD-induced macropinocytotic fluid-phase uptake is intact in glycan-deficient cells. A, parental CHO and glycan mutant cells treated with unlabeled TAT PTD peptide in the presence of 70-kDa neutral dextran-Texas Red for 1 h at 37 °C. After washing and trypsinization, cells were assayed for dextran uptake by FACS. B, live cell photomicrographs of the indicated cell lines following treatment with 1 μ
m
TAT PTD in the presence of 70-kDa dextran-TMR. C, TAT PTD-induced uptake of 70-kDa dextran-Texas Red following treatment with 200 milliunits of heparinase for 1 h at 37 °C. The TAT PTD concentration was 1 μ
m
. D, cells treated with 200 milliunits of sialidase followed by dextran and TAT PTD as described for C. WT, wild-type.
FIGURE 6.
Enzymatic depletion of HS and SAs does not impair TAT PTD transduction into parental or glycan-deficient cells or murine T cells. A and B, the indicated Lox-STOP-Lox-GFP stable cell lines were treated for 1 h with heparinase or sialidase as indicated at 37 °C, followed by 1 μ
m
TAT PTD-Cre for 1 h at 37 °C, trypsinization, and replating. GFP expression was assayed by flow cytometry 24 h after TAT PTD-Cre treatment. C and D, the indicated cell lines were treated with heparinase and sialidase enzymes as described for A and B, followed by treatment with 1 μ
m
TAT PTD-FITC peptide for 1 h at 37 °C, immediately followed by PBS, heparin, and trypsin washes and flow cytometry. E, heparinase treatment does not affect transduction efficiency in the Tex-LoxP-EGFP murine thymoma cell line. WT, wild-type.
Similar articles
- Tat(48-60) peptide amino acid sequence is not unique in its cell penetrating properties and cell-surface glycosaminoglycans inhibit its cellular uptake.
Subrizi A, Tuominen E, Bunker A, Róg T, Antopolsky M, Urtti A. Subrizi A, et al. J Control Release. 2012 Mar 10;158(2):277-85. doi: 10.1016/j.jconrel.2011.11.007. Epub 2011 Nov 12. J Control Release. 2012. PMID: 22100438 - Antennapedia and HIV transactivator of transcription (TAT) "protein transduction domains" promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans.
Console S, Marty C, García-Echeverría C, Schwendener R, Ballmer-Hofer K. Console S, et al. J Biol Chem. 2003 Sep 12;278(37):35109-14. doi: 10.1074/jbc.M301726200. Epub 2003 Jun 30. J Biol Chem. 2003. PMID: 12837762 - Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters.
Ziegler A, Seelig J. Ziegler A, et al. Biophys J. 2004 Jan;86(1 Pt 1):254-63. doi: 10.1016/S0006-3495(04)74101-6. Biophys J. 2004. PMID: 14695267 Free PMC article. - Intracellular transduction and potential of Tat PTD and its analogs: from basic drug delivery mechanism to application.
Zhang X, Zhang X, Wang F. Zhang X, et al. Expert Opin Drug Deliv. 2012 Apr;9(4):457-72. doi: 10.1517/17425247.2012.663351. Expert Opin Drug Deliv. 2012. PMID: 22432469 Review. - Progress in Research and Application of HIV-1 TAT-Derived Cell-Penetrating Peptide.
Zou L, Peng Q, Wang P, Zhou B. Zou L, et al. J Membr Biol. 2017 Apr;250(2):115-122. doi: 10.1007/s00232-016-9940-z. Epub 2016 Dec 8. J Membr Biol. 2017. PMID: 27933338 Review.
Cited by
- Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis.
Cafaro A, Schietroma I, Sernicola L, Belli R, Campagna M, Mancini F, Farcomeni S, Pavone-Cossut MR, Borsetti A, Monini P, Ensoli B. Cafaro A, et al. Int J Mol Sci. 2024 Jan 30;25(3):1704. doi: 10.3390/ijms25031704. Int J Mol Sci. 2024. PMID: 38338977 Free PMC article. Review. - A membrane penetrating peptide aptamer inhibits STAT3 function and suppresses the growth of STAT3 addicted tumor cells.
Borghouts C, Delis N, Brill B, Weiss A, Mack L, Lucks P, Groner B. Borghouts C, et al. JAKSTAT. 2012 Jan 1;1(1):44-54. doi: 10.4161/jkst.18947. JAKSTAT. 2012. PMID: 24058750 Free PMC article. - The TAT Protein Transduction Domain as an Intra-Articular Drug Delivery Technology.
Mailhiot SE, Thompson MA, Eguchi AE, Dinkel SE, Lotz MK, Dowdy SF, June RK. Mailhiot SE, et al. Cartilage. 2021 Dec;13(2_suppl):1637S-1645S. doi: 10.1177/1947603520959392. Epub 2020 Sep 19. Cartilage. 2021. PMID: 32954793 Free PMC article. - Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics.
Lönn P, Kacsinta AD, Cui XS, Hamil AS, Kaulich M, Gogoi K, Dowdy SF. Lönn P, et al. Sci Rep. 2016 Sep 8;6:32301. doi: 10.1038/srep32301. Sci Rep. 2016. PMID: 27604151 Free PMC article. - Labeling and in vivo visualization of transplanted adipose tissue-derived stem cells with safe cadmium-free aqueous ZnS coating of ZnS-AgInS2 nanoparticles.
Ogihara Y, Yukawa H, Kameyama T, Nishi H, Onoshima D, Ishikawa T, Torimoto T, Baba Y. Ogihara Y, et al. Sci Rep. 2017 Jan 6;7:40047. doi: 10.1038/srep40047. Sci Rep. 2017. PMID: 28059135 Free PMC article.
References
- Meade B. R., Dowdy S. F. (2007) Adv. Drug Delivery Rev. 59, 134–140 - PubMed
- El-Andaloussi S., Holm T., Langel U. (2005) Curr. Pharm. Des. 11, 3597–3611 - PubMed
- Goun E. A., Pillow T. H., Jones L. R., Rothbard J. B., Wender P. A. (2006) ChemBioChem 7, 1497–1515 - PubMed
- Gump J. M., Dowdy S. F. (2007) Trends Mol. Med. 13, 443–448 - PubMed
- Nakase I., Takeuchi T., Tanaka G., Futaki S. (2008) Adv. Drug Delivery Rev. 60, 598–607 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources