Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract - PubMed (original) (raw)
Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract
Zeynep Firtina et al. J Biol Chem. 2009.
Abstract
Human diseases caused by mutations in extracellular matrix genes are often associated with an increased risk of cataract and lens capsular rupture. However, the underlying mechanisms of cataract pathogenesis in these conditions are still unknown. Using two different mouse models, we show that the accumulation of collagen chains in the secretory pathway activates the stress signaling pathway termed unfolded protein response (UPR). Transgenic mice expressing ectopic Col4a3 and Col4a4 genes in the lens exhibited activation of IRE1, ATF6, and PERK associated with expansion of the endoplasmic reticulum and attenuation of general protein translation. The expression of the transgenes had adverse effects on lens fiber cell differentiation and eventually induced cell death in a group of transgenic fiber cells. In Col4a1(+/Deltaex40) mutant mice, the accumulation of mutant chains also caused low levels of UPR activation. However, cell death was not induced in mutant lenses, suggesting that low levels of UPR activation are not proapoptotic. Collectively, the results provide in vivo evidence for a role of UPR in cataract formation in response to accumulation of terminally unfolded proteins in the endoplasmic reticulum.
Figures
FIGURE 1.
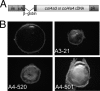
The ectopic expression of Col4a3 or Col4a4 transgenes in the embryonic mouse lens results in lens opacity and microphthalmia. A, diagram of the transgene vector. The chick δ1-crystallin enhancer (δ_en_) fused to a mouse short αA-crystallin promoter (α_A_) was used to drive the expression (43). The cDNAs for the transgenes were placed between the rabbit β-globin intron (640 bp) and the human growth hormone poly(A) signal (pA; 660 bp). B, dark field images of the 2.5 months old WT and transgenic lenses. Both Col4a4 lines (bottom) and one of the Col4a3 lines (top right) had significantly smaller eyes with obvious opacities and other lens defects. A4-520 and A4-501, two Col4a4 lines; A3-21, Col4a3 line.
FIGURE 2.
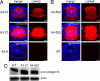
Exogenous COL4A3 or COL4A4 chains accumulate to high levels in affected transgenic fiber cells. A, a chain-specific antibody against COL4A3 was used to detect the expression in the affected A3-21 (top and middle) and the unaffected A3-9 (bottom) lenses. At E12.5, Col4a3 transgene is highly expressed in the affected A3-21 fiber cells, whereas there is no detectable expression in the unaffected A3-9 lenses at this age. B, a chain-specific antibody against COL4A4 was used to detect its expression in the affected A4-520 (top and middle) and the WT (bottom) lenses. At E12.5, Col4a4 transgene is highly expressed in the affected A4-520 fiber cells, whereas there is no detectable expression in the wild type lenses at this age. The gain for the middle and bottom panels in both A and B was increased ∼25% to obtain an overexposed image showing possible secretion of exogenous collagen chains. The arrowheads indicate the detection of secreted collagen chains around the lens. Bars, 77 μm; e, epithelium; f, fiber cells. Red, collagen IV chain; blue, DNA. C, expression of collagen IV chains in E14.5 lenses was detected by Western blotting using a pan-collagen IV antibody. The affected A4-520 and A3-21 lenses have elevated levels of soluble collagen IV chains.
FIGURE 3.
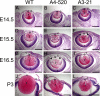
Lens histology was compared between WT (A, D, G, and J), A4-520 (B, E, H, and K), and A3-21 (C, F, I, and L) lenses at different developmental stages. At E14.5, A4-520 (B) and A3-21 (C) fiber cells are in contact the epithelium as in WT lenses (A). At E15.5, line A3-21 (F) fiber cells lose contact with the epithelium, whereas WT (D) and A4-520 (E) fibers look normal. At E16.5, A4-520 (H) fiber cells also lose contact with the epithelium, whereas WT (G) fibers grow normally. After birth, A4-520 (K) and A3-21 (L) lenses were significantly smaller with major lens defects. The arrowheads indicate the apical tips of improperly elongated fiber cells. Scale bar, 330 μm; c, cornea; e, epithelium; f, fiber cells; r, retina. Pink, cytoplasm; purple, cell nuclei.
FIGURE 4.
Scanning electron microscopy analysis of the WT and A4-520 fiber cells. Fiber cells from transgenic mice (bottom) are disorganized with irregular interlocking junctions as compared with the regular organization of the WT lens fiber cells (top). Scale bar, 10 μm.
FIGURE 5.
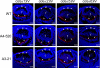
Secretion of COL4A1, -A2, -A5, and -A6 chains is not affected in the transgenic fiber cells. Chain-specific antibodies against COL4A1, -A2, -A5, and -A6 were used to detect their expression in WT (top), A4-520 (middle), and A3-21 (bottom) lenses. The arrowheads indicate the secreted collagen IV chains incorporated into the lens capsule. Bars, 77 μm; e, epithelium; f, fiber cells. Blue, DNA; red, collagen IV chain.
FIGURE 6.
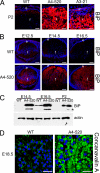
The endoplasmic reticulum in affected transgenic lenses is greatly extended with an up-regulation of the major ER chaperone BiP. A, the expression of BiP (red) in 2-day-old WT (left), A4-520 (middle), and A3-21 (right) lenses detected by immunofluorescence. The two affected transgenic lines (A4-520 and A3-21) show much higher levels of BiP expression in lens fiber cells compared with WT lens. B, the expression of BiP (red) in WT (top) and A4-520 (bottom) lenses detected by immunofluorescence. In the A4-520 lens, BiP expression starts to up-regulate at E12.5. Bars, 77 μm; e, epithelium; f, fiber cells. C, the expression of BiP in WT and A4-520 lenses detected by Western blotting. BiP protein is found at higher levels in E14.5 transgenic lenses and remains elevated after birth. The asterisks indicate the smaller 72 kDa band detected by BiP antibody. This band was previously proposed to be a degradation product of wild type BiP (95). WT lenses also express two BiP bands upon longer exposure (not shown). D, endoplasmic reticulum was probed in WT (left) and A4-520 (right) lenses using concanavalin A (green). Note that the affected A4-520 fiber cells have much higher staining around the nuclei and throughout the cytoplasm. Bars, 40 μm; e, epithelium; f, fiber cells. In all panels, blue labels DNA.
FIGURE 7.
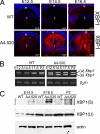
The IRE1/Xbp1 pathway is activated in transgenic lenses. A, expression of XBP1 (both spliced and unspliced) in WT (top) and A4-520 (bottom) lenses detected by immunofluorescence. The level of XBP1 expression in the A4-520 lens is up-regulated at E12.5 and increases in time. Bars, 77 μm; e, epithelium; f, fiber cells. Blue, DNA; red, XBP1. B, RT-PCR analysis of Xbp1 splicing. The spliced Xbp1 mRNA is detected in A4-520 lenses starting at E12.5. β2-Microglobulin was used as a control. C, immunoblot analysis of XBP1 protein in WT and A4-520 lenses. Note that the protein produced from spliced Xbp1 is only found in the affected A4-520 lens, whereas protein from unspliced Xbp1 is produced both in WT and A4-520 lenses. Actin was used as a loading control.
FIGURE 8.
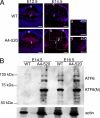
ATF6 pathway is activated in transgenic lenses. A, expression of ATF6 in WT (top) and A4-520 (bottom) lenses detected by immunofluorescence. Note that the level of ATF6 expression in the A4-520 lens is up-regulated at E12.5 and remains elevated. The ×40 magnification images (_40_×) (x and y) on the right show the boxed regions from transgenic lenses. Bars, 77 μm; e, epithelium; f, fiber cells. Blue, DNA; red, ATF6. B, immunoblot analysis of ATF6 protein in WT and A4-520 lenses. Note that at E14.5, ATF6 protein is not detectable in WT lenses, but both forms (full-length/ER-resident and cleaved/nuclear) of ATF6 are highly expressed in A4-520 lenses. Actin was used as a loading control. ATF6(N), nuclear/cleaved form of ATF6.
FIGURE 9.
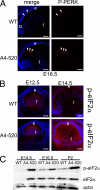
PERK/eIF2α pathway is activated in transgenic lenses. A, expression of phospho-PERK (red) in WT (top) and A4-520 (bottom) lenses detected by immunofluorescence. Note that phospho-PERK levels are increased at the apical tips of A4-520 transgenic fiber cells at E16.5. The arrowheads indicate the phospho-PERK detected at the apical tips of normal fiber cells (top) and transgenic fiber cells that lost contact with the epithelium (bottom). B, expression of phospho-eIF2α (red) in WT (top) and A4-520 (bottom) lenses detected by immunofluorescence. Note that phospho-eIF2α levels are initially up-regulated in A4-520 lenses at E12.5 and continue to increase with time. Bars, 77 μm; e, epithelium; f, fiber cells. In all panels, blue labels DNA. C, immunoblot analysis of eIF2α protein in WT and A4-520 lenses. Note that at E14.5, both total and phospho-eIF2α are not detectable in the WT lens, but they are present at high levels in A4-520 lenses. Also note that total eIF2α levels are similar in WT and A4-520 lenses at E16.5 and P2, whereas phospho-eIF2α levels are elevated in the A4-520 lens. Actin was used as a loading control.
FIGURE 10.
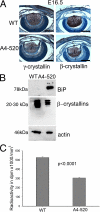
Protein Translation is inhibited in the transgenic lenses. A, expression of γ (left) and β crystallins (right) in WT (top) and A4-520 lenses (bottom) detected by immunohistochemistry. At E16.5, A4-520 lenses have less crystallin expression as compared with WT lenses. Bars, 330 μm. c, cornea; e, epithelium; f, fiber cells; r, retina. Blue, DNA; brown, crystallins. B, 1% of lens soluble proteins were subjected to SDS-PAGE, and the expression of BiP, β-crystallins, and actin was detected by immunoblotting. In 1% of lens soluble proteins, compared with WT lenses, A4-520 lenses have much higher levels of BiP protein and lower levels of β-crystallins, whereas actin levels are similar. C, protein translation rate was measured in WT and A4-520 lenses by incubating freshly isolated E17.5 lenses in Medium 199 supplemented with 3H-labeled amino acid mixture. The y axis shows the radioactivity measured in dpm/ml3 of lens volume. The bars represent the average radioactivity measured in WT and A4-520 lenses. Statistical analysis was done using Student's t test. p < 0.0001.
FIGURE 11.
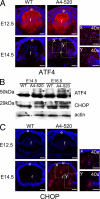
ATF4 and its target CHOP are expressed by the transgenic fiber cells. A, expression of ATF4 (red) in WT (left) and A4-520 (right) lenses detected by immunofluorescence. At E12.5, ATF4 is present in the cytoplasm of both normal and transgenic lenses. However, at E14.5, transgenic fiber cells exhibit elevated levels of nuclear ATF4 in fiber cells. The ×40 magnification images (_40_×) (x and y) on the right show the boxed regions from transgenic lenses. B, immunoblot analysis of ATF4 and CHOP expression. ATF4 was detected as two bands. The lower band was abundantly produced in both normal and transgenic lenses, whereas the 50 kDa band (marked with an asterisk) was found only in the transgenics. The 29-kDa CHOP (marked with an asterisk) was only found in the transgenic lenses. C, expression of CHOP (red) in WT (left) and A4-520 (right) lenses detected by immunofluorescence. At E12.5, there is a very weak CHOP expression in transgenic fiber cells. At E14.5, a group of fiber cells express CHOP strongly in their nuclei. The ×40 magnification images (_40_×) (x and y) on the right show the boxed regions from transgenic lenses. Bars, 77 μm; e, epithelium; f, fiber cells. In all panels, blue labels DNA.
FIGURE 12.
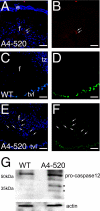
Apoptosis is induced in some transgenic fiber cells at E18.5. A and B, cleaved caspase 3 was detected in a group of central fiber cells by immunofluorescence. A, an antibody specific to the cleaved form of caspase 3 (red) was used to detect the activation of this caspase in E18.5 transgenic lenses. Note the red staining in A4-520 transgenic fiber cells indicated with arrows. B, cleaved caspase 3 staining (red) without the nuclear counterstain. C–E, apoptotic cells were detected by TUNEL staining. C, TUNEL staining (green) of E18.5 WT lenses. Note that no WT lens cells are found to be TUNEL-positive, whereas the blood vessels behind the lens (tunica vasculosa lentis) are positively stained. D, TUNEL staining (green) of WT lenses without the nuclear stain. E, TUNEL staining (green) of E18.5 A4-520 lenses. A group of central A4-520 fiber cells (indicated by arrows) are stained TUNEL-positive. F, TUNEL staining (green) of A4-520 lenses without the nuclear stain. In all panels, blue labels DNA. Bars, 77 μm. e, epithelial cells, f, fiber cells; tvl, tunica vasculosa lentis. G, activation of caspase-12 in E18.5 transgenic lenses examined by Western blot analysis. Asterisks, active forms of caspase-12 detected with anti-caspase-12 antibody.
FIGURE 13.
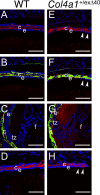
Mutant COL4A1 accumulates in Col4a1+/exΔ40 lenses along with its binding partner COL4A2. Chain-specific antibodies against COL4A1, laminin, and COL4A2 were used to detect these proteins in WT (left) and Col4a1+/exΔ40 lenses (right). At E18.5, COL4A1 (A–C, red), laminin (B and C, green), and COL4A2 (D, red) are secreted into the lens capsule of normal lenses. In mutant lenses, COL4A1 accumulates inside mutant Col4a1+/exΔ40 lens epithelial (E and F, red) and fiber cells (G, red). Similarly, COL4A2 also partially accumulates inside mutant lens cells (H). The arrowheads indicate the epithelial cells that retain collagen chains in mutant lenses. Bars, 77 μm. c, lens capsule; e, epithelium; f, fiber cells; tz, transition zone. Blue, DNA; red, collagen chains; green, pan-laminin.
FIGURE 14.
UPR is activated in the Col4a1+/exΔ40 lenses. A, expression of BiP (red) in WT (left) and Col4a1+/exΔ40 (right) lenses detected by immunofluorescence. At E18.5, BiP is up-regulated in mutant lens epithelium and fiber cells. B, expression of ATF6 (red) in WT (left) and Col4a1+/exΔ40 (right) lenses detected by immunofluorescence. ATF6 is up-regulated in mutant lens epithelium and fiber cells. The ×40 magnification images (_40_×) (x and y) at the bottom panels show the boxed regions from the top panels. C, expression of XBP1 (red) in WT (left) and Col4a1+/exΔ40 (right) lenses detected by immunofluorescence. XBP1 up-regulation is detected in parts of the epithelium. D, RT-PCR analysis of Xbp1 splicing in WT and Col4a1+/exΔ40 lenses. The splicing of Xbp1 is induced in the mutant lenses. E, expression of phospho-eIF2α (red) in Col4a1+/exΔ40 lenses detected by immunofluorescence. Note that phospho-eIF2α is expressed by some central mutant fiber cells at E18.5. The ×40 magnification image (_40_×) (x) on the right shows the boxed regions from Col4a1+/exΔ40 lenses on the left. Bars, 77 μm; e, epithelium; f, fiber cells. In all panels, blue labels DNA.
FIGURE 15.
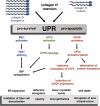
UPR is activated in the lenses of transgenic and mutant mice upon accumulation of collagen chains. In transgenics, exogenous expression of Col4a3 or Col4a4 genes in the embryonic lens causes high levels of collagen chain accumulation inside the transgenic fiber cells and results in immediate activation of all three known UPR sensors. In Col4a1+/exΔ40 mice, mutant COL4A1 chains cannot properly assemble and accumulate in lens cells along with COL4A2 and activate all three UPR sensors. In transgenics, the translational attenuation induced upon eIF2α phosphorylation negatively affects the synthesis and accumulation of major lens proteins, crystallins. The phosphorylation of eIF2α also results in selective translation of ATF4 and induction of its target CHOP in transgenic lenses, whereas in mutants, this response has not been detected (marked with an asterisk). Transgenic lens fiber cells experiencing UPR activation exhibited defective fiber cell elongation around E15.5 and cell death later in E18.5. In contrast, cell death was not observed in mutants (marked with an asterisk). Overall, the data suggest that all UPR responses could contribute to the lens pathology observed, including inhibition of denucleation, disruption of lens ultrastructure, opacity, and microphthalmia.
Similar articles
- Activation of the unfolded protein response in aged human lenses.
Tang HZ, Yang LM. Tang HZ, et al. Mol Med Rep. 2015 Jul;12(1):389-93. doi: 10.3892/mmr.2015.3417. Epub 2015 Mar 4. Mol Med Rep. 2015. PMID: 25739021 - Plasma cell differentiation initiates a limited ER stress response by specifically suppressing the PERK-dependent branch of the unfolded protein response.
Ma Y, Shimizu Y, Mann MJ, Jin Y, Hendershot LM. Ma Y, et al. Cell Stress Chaperones. 2010 May;15(3):281-93. doi: 10.1007/s12192-009-0142-9. Epub 2009 Nov 8. Cell Stress Chaperones. 2010. PMID: 19898960 Free PMC article. - PERK regulated miR-424(322)-503 cluster fine-tunes activation of IRE1 and ATF6 during Unfolded Protein Response.
Gupta A, Hossain MM, Read DE, Hetz C, Samali A, Gupta S. Gupta A, et al. Sci Rep. 2015 Dec 17;5:18304. doi: 10.1038/srep18304. Sci Rep. 2015. PMID: 26674075 Free PMC article. - Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.
Yu M, Lun J, Zhang H, Wang L, Zhang G, Zhang H, Fang J. Yu M, et al. Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review. - Contribution of the Unfolded Protein Response (UPR) to the Pathogenesis of Proteasome-Associated Autoinflammatory Syndromes (PRAAS).
Ebstein F, Poli Harlowe MC, Studencka-Turski M, Krüger E. Ebstein F, et al. Front Immunol. 2019 Nov 26;10:2756. doi: 10.3389/fimmu.2019.02756. eCollection 2019. Front Immunol. 2019. PMID: 31827472 Free PMC article. Review.
Cited by
- COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke.
Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, Valant V, Greenberg SM, Rosand J, Gould DB. Jeanne M, et al. Am J Hum Genet. 2012 Jan 13;90(1):91-101. doi: 10.1016/j.ajhg.2011.11.022. Epub 2011 Dec 29. Am J Hum Genet. 2012. PMID: 22209247 Free PMC article. - COL4A2 mutation associated with familial porencephaly and small-vessel disease.
Verbeek E, Meuwissen ME, Verheijen FW, Govaert PP, Licht DJ, Kuo DS, Poulton CJ, Schot R, Lequin MH, Dudink J, Halley DJ, de Coo RI, den Hollander JC, Oegema R, Gould DB, Mancini GM. Verbeek E, et al. Eur J Hum Genet. 2012 Aug;20(8):844-51. doi: 10.1038/ejhg.2012.20. Epub 2012 Feb 15. Eur J Hum Genet. 2012. PMID: 22333902 Free PMC article. - Biology of Inherited Cataracts and Opportunities for Treatment.
Shiels A, Hejtmancik JF. Shiels A, et al. Annu Rev Vis Sci. 2019 Sep 15;5:123-149. doi: 10.1146/annurev-vision-091517-034346. Annu Rev Vis Sci. 2019. PMID: 31525139 Free PMC article. Review. - Deficiency of Yes-Associated Protein Induces Cataract in Mice.
He Q, Gao Y, Wang T, Zhou L, Zhou W, Yuan Z. He Q, et al. Aging Dis. 2019 Apr 1;10(2):293-306. doi: 10.14336/AD.2018.0910. eCollection 2019 Apr. Aging Dis. 2019. PMID: 31011480 Free PMC article. - A simple method for quantitating confocal fluorescent images.
Shihan MH, Novo SG, Le Marchand SJ, Wang Y, Duncan MK. Shihan MH, et al. Biochem Biophys Rep. 2021 Feb 1;25:100916. doi: 10.1016/j.bbrep.2021.100916. eCollection 2021 Mar. Biochem Biophys Rep. 2021. PMID: 33553685 Free PMC article.
References
- Congdon N., Vingerling J. R., Klein B. E., West S., Friedman D. S., Kempen J., O'Colmain B., Wu S. Y., Taylor H. R. (2004) Arch. Ophthalmol. 122, 487–494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY015279/EY/NEI NIH HHS/United States
- P20 RR016472/RR/NCRR NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
- EY02162/EY/NEI NIH HHS/United States
- P20 RR16472/RR/NCRR NIH HHS/United States
- R01 EY015279/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases