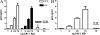Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells - PubMed (original) (raw)
. 2009 Nov 23;206(12):2717-33.
doi: 10.1084/jem.20091579. Epub 2009 Oct 26.
Federico Remes Lenicov, Juan Sabatté, Christian Rodríguez Rodrígues, Mercedes Cabrini, Carolina Jancic, Silvina Raiden, Mónica Donaldson, Rodolfo Agustín Pasqualini Jr, Clara Marin-Briggiler, Mónica Vazquez-Levin, Francisco Capani, Sebastián Amigorena, Jorge Geffner
Affiliations
- PMID: 19858326
- PMCID: PMC2806607
- DOI: 10.1084/jem.20091579
Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells
Ana Ceballos et al. J Exp Med. 2009.
Abstract
Semen is the main vector for HIV-1 dissemination worldwide. It contains three major sources of infectious virus: free virions, infected leukocytes, and spermatozoa-associated virions. We focused on the interaction of HIV-1 with human spermatozoa and dendritic cells (DCs). We report that heparan sulfate is expressed in spermatozoa and plays an important role in the capture of HIV-1. Spermatozoa-attached virus is efficiently transmitted to DCs, macrophages, and T cells. Interaction of spermatozoa with DCs not only leads to the transmission of HIV-1 and the internalization of the spermatozoa but also results in the phenotypic maturation of DCs and the production of IL-10 but not IL-12p70. At low values of extracellular pH (approximately 6.5 pH units), similar to those found in the vaginal mucosa after sexual intercourse, the binding of HIV-1 to the spermatozoa and the consequent transmission of HIV-1 to DCs were strongly enhanced. Our observations support the notion that far from being a passive carrier, spermatozoa acting in concert with DCs might affect the early course of sexual transmission of HIV-1 infection.
Figures
Figure 1.
Capture of HIV-1 by human spermatozoa. Spermatozoa (1.5 × 106/200 µl) were incubated with different amounts of HIV-1 BAL (R5-tropic; A) or HIV-1 IIIB (X4-tropic; B) for 60 min at 37 or 4°C. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of six to eight experiments performed in triplicate. In A, spermatozoa was cultured with HIV-1 BAL containing 75 ng p24 for 60 min at 37°C, washed thoroughly, treated with 1,000 U/ml trypsin for 15 min at 37°C, lysed, and assayed for p24 antigen by ELISA. (−) sp represents the values of p24 found in wells in which spermatozoa were omitted (nonspecific attachment of HIV-1 to the wells). The asterisk represents statistical significance (P < 0.05 vs. untreated spermatozoa incubated with 75 ng HIV-1 BAL).
Figure 2.
MRs do not play a major role in the capture of HIV-1 by spermatozoa. (A) Spermatozoa were treated with 1,000 U/ml trypsin or 20 µg/ml pronase for 15 min at 37°C, washed thoroughly, and 1.5 × 106/200 µl were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C. Cells were then washed, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of five experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (B) The expression of MRs in the spermatozoa was analyzed by measuring the binding of albumin bovine-α-
d
-mannopyranosylphenyl isothiocyanate-BMA revealed by streptavidin-FITC. Assays were performed by incubating spermatozoa for 30 min at 37°C with 100 µg/ml BMA in the absence or presence of 5 mg/ml mannan. The gray histogram represents spermatozoa incubated only with streptavidin-FITC (isotype). A representative experiment (n = 4) is shown. (C) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C in the absence or presence of mannan, mannose-BSA, or blocking antibodies directed to MR. Cells were washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of four to eight experiments performed in duplicate. (D) Human macrophages (105/100 µl), obtained from monocytes cultured with GM-CSF for 5 d, were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C in the absence or presence of mannan, mannose-BSA, or blocking antibodies directed to MR. Cells were washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of three to four experiments performed in duplicate. (E) B-THP-1-DC-SIGN+ cells (2.5 × 105/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C in the absence or presence of mannan or mannose-BSA, washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of three to five experiments performed in duplicate. Asterisks in C–E represent statistical significance (P < 0.05 vs. controls). The capture mediated by THP-1 cells, which do not express DC-SIGN, is also shown (black bar).
Figure 3.
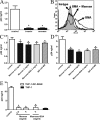
Capture of HIV-1 by spermatozoa is mainly mediated through HS. (A) The expression of HS on spermatozoa was analyzed by flow cytometry using anti–HS-specific antibodies (clone 10E4). Isotype control (gray histogram) and 10E4 labeled (open histogram) are depicted. A representative experiment (n = 7) is shown. (B) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng of p24 for 60 min at 37°C in the absence or presence of 10 and 100 U/ml heparin, washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of six experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (C) Spermatozoa were treated with 5 U/ml heparinase II for 60 min at 25°C or 1,000 U/ml trypsin for 15 min at 37°C. Then the expression of HS was analyzed by flow cytometry. The results are expressed as the MFI ± SEM of five experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. the expression of HS in controls). (D) Spermatozoa were treated with 1 and 5 U/ml heparinase II for 60 min at 25°C. Then their ability to capture HIV-1 was assayed as described for Fig. 2 A. Results are the mean ± SEM of five experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (E) Spermatozoa were treated with 5 U/ml heparinase III for 60 min at 25°C. Then the expression of the neoepitope 3G10 was analyzed by flow cytometry. Isotype (gray histogram) and 3G10-labeled untreated (control) or heparinase III–treated spermatozoa (open histograms) are shown. Isotype controls were similar for untreated and heparinase III–treated spermatozoa. A representative experiment (n = 4) is shown. (F) Spermatozoa (1.5 × 106/200 µl) were incubated with different primary HIV-1 isolates containing 25 ng of p24 for 60 min at 37°C in the absence or presence of 100 U/ml heparin or 5 mg/ml mannan, washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of three to five experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (G) HIV-1 pseudotypes were produced as described in Materials and methods. Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 env− or HIV-1 env+ pseudotypes containing 40 ng of p24 for 60 min at 37°C in the absence or presence of 5 mg/ml mannan or 100 U/ml heparin, washed thoroughly, lysed, and assayed for p24 antigen by ELISA. Results are the mean ± SEM of three to four experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (H) The expression of syndecans 1–4 was analyzed by flow cytometry. Gray histograms correspond to isotype controls. In each case, a representative experiment (n = 3–6) is shown.
Figure 4.
Spermatozoa efficiently transmit HIV-1 to DCs, macrophages, and CD4+ T cells. (A) DCs were incubated for 60 min at 37°C with HIV-1 containing 25 ng of p24, in the absence (gray bar) or presence of different numbers of spermatozoa (Sp; open bars). Cells were then washed four times to remove free virus, and the infection of DCs was revealed after 7 d by measuring the amount of p24 in cell supernatants. Black bars represent spermatozoa incubated alone with HIV-1 containing 25 ng p24. Results are the mean ± SEM of four experiments performed in duplicate. Asterisk represents statistical significance (P < 0.05 vs. DCs). (B) DCs (105/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C. Cells were then washed thoroughly to remove free virus and then DCs were cultured with or without 1.5 or 6.0 × 106 spermatozoa. Infection of DCs was revealed after 7 d. The results are expressed as the mean ± SEM of four experiments performed in duplicate. (C) Spermatozoa suspended at different concentrations were incubated with HIV-1 BAL containing 25 ng p24/200 µl of spermatozoa suspension for 60 min at 37°C. Cells were then washed thoroughly, different numbers of HIV-1–treated spermatozoa were incubated with (open bars) or without DCs (black bars) over 7 d, and the infection of DCs was then analyzed. Results are the mean ± SEM of four to five experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. spermatozoa cultured without DCs). (D) Spermatozoa (1.5 × 106/200 µl) were incubated with the primary HIV-1 isolates 93BR020.1 or GARR-G4 containing 25 ng p24 for 60 min at 37°C, and then cells were washed thoroughly. Spermatozoa and DCs were co-cultured during 7 d at a spermatozoa/DC ratio of 10:1, and the infection of DCs was then analyzed. Results are the mean ± SEM of three experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (E) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C, washed thoroughly, and co-cultured with 105 DCs during 3, 7, and 12 d at a spermatozoa/DC ratio of 10:1. Infection of DCs was then analyzed. Black bars represent the amount of p24 found in the supernatants of HIV-1–treated spermatozoa cultured without DCs. Results are the mean ± SEM of four experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. controls). (F) Spermatozoa (1.5 × 106 /200 µl) were treated with 5 U/ml heparinase II for 60 min at 25°C, incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C, washed thoroughly, and co-cultured during 7 d with DCs at a spermatozoa/DC ratio of 10:1. Infection of DCs was then revealed as described in A. Black bars represent the levels of p24 antigen in the supernatants of HIV-1–treated spermatozoa cultured alone. Results are the mean ± SEM of four experiments performed in duplicate. The asterisk represents statistical significance (P < 0.05 vs. controls). (G) Spermatozoa (1.5 × 106/200 µl) were incubated with 25 ng HIV-1 BAL or HIV-1 IIIB for 60 min at 37°C. Cells were then washed thoroughly and incubated with macrophages, the T cell line MT2, PHA plus IL-2–activated PBMCs, or purified CD3+ T cells activated by PHA plus IL-2 (1.5 × 105 cells/200 µl). Spermatozoa treated with HIV-1 BAL were used in macrophage cultures, whereas spermatozoa treated with HIV-1 IIIB were used for MT2 cells, PBMCs, and purified T cells. Cellular infection was analyzed after 7 d of culture. Results are the mean ± SEM of three to four experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. spermatozoa cultured alone). (H) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 IIIB containing 25 ng of p24 for 60 min at 37°C, and then cells were washed thoroughly. Spermatozoa and DCs were co-cultured during 60 min at 37°C at a spermatozoa/DC ratio of 10:1. Cells were then treated with 1,000 U/ml trypsin for 15 min at 37°C, washed, and cultured with or without 3.0 × 105 T cells purified from peripheral blood and activated with PHA plus IL-2. The amount of p24 antigen in cell supernatants was analyzed after 7 d of culture. Results are the mean ± SEM of three experiments performed in duplicate. The asterisk represents statistical significance (P < 0.05 vs. Sp + DCs).
Figure 5.
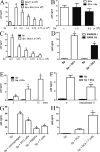
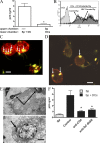
Spermatozoa strongly interact with DCs. (A) Spermatozoa (5 × 106/400 µl) were incubated with 50 ng HIV-1 BAL for 60 min at 37°C. Cells were then washed thoroughly. Experiments were performed in 24-transwell plates with a polycarbonate filter (0.2-µm pore size). 5 × 106 HIV-1–treated spermatozoa were in the upper compartment and 5 × 105 DCs were seeded in the lower compartment. Controls were performed by incubating together HIV-1–treated spermatozoa and DCs in the lower compartment. Cells were cultured for 7 d and the infection of DCs was evaluated by measuring the amount of p24 in the supernatants of DC cultures. Results are the mean ± SEM of five experiments performed in triplicate. The asterisk represents statistical significance (P < 0.05 vs. controls). (B) CFSE-labeled spermatozoa (1.5 × 106/200 µl) were incubated with or without HIV-1 BAL containing 25 ng p24 for 60 min at 37°C. Cells were then washed thoroughly and were incubated with unlabeled DCs at a spermatozoa/DC ratio of 10:1 during 1 h at 37°C. The interaction of spermatozoa and DCs were then analyzed by flow cytometry in the gate of DCs, which could be easily distinguished from spermatozoa because of their higher values of forward light scatter. Histograms from a representative experiment (n = 7) are shown. (C and D) CFSE-labeled spermatozoa (1.5 × 106/200 µl) were incubated during 60 min at 37°C with DCs, previously labeled with PE–anti–HLA-DR antibodies, at a spermatozoa/DC ratio of 10:1. The interaction of spermatozoa and DCs were then analyzed by fluorescence microscopy (C) or laser confocal microscopy (D). Bars, 10 µm. (E) Spermatozoa (1.5 × 106/200 µl) were incubated during 60 min at 37°C with DCs at a spermatozoa/DC ratio of 10:1. The interaction of spermatozoa and DCs were then analyzed by electron microscopy. Representative images are shown. Arrows in C–E indicate spermatozoa attached to or ingested by DCs. Bars: (top) 0.5 µm; (bottom) 1.5 µm. (F) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C and washed thoroughly. DCs were pretreated, or not, with blocking antibodies directed to either CD4 or DC-SIGN for 15 min at 4°C. Blocking antibodies were used at a concentration three- to fivefold higher than those needed to saturate all binding sites, as determined by FACS analysis. Spermatozoa and DCs were co-cultured during 7 d at a spermatozoa/DC ratio of 10:1, and the infection of DCs was then analyzed by measuring the levels of p24 antigen in cell supernatants. Also shown in the figure is the amount of p24 found in the supernatants of HIV-1–treated spermatozoa cultured for 7 d without DCs (gray bar). The results are expressed as the mean ± SEM of four experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. controls).
Figure 6.
Interaction with spermatozoa leads to the phenotypic maturation of DCs and the production of IL-10. (A) Representative histograms of the phenotype of immature DCs. Gray histograms represent isotype controls. (B) Spermatozoa (Sp; 1.5 × 106/200 µl) were incubated for 24 h at 37°C with DCs at a spermatozoa/DC ratio of 10:1, and the phenotype of DCs was then analyzed by flow cytometry. The phenotype of DCs incubated alone or in the presence of spermatozoa or 100 ng/ml LPS during 24 h at 37°C is also shown. Results are expressed as MFI values and represent the arithmetic mean ± SEM of 11 experiments. Asterisks represent statistical significance (P < 0.05 vs. controls). (C and D) The production of IL-10 and IL-12p70 was evaluated in cell supernatants of DCs cultured alone or in the presence of spermatozoa or 100 ng/ml LPS during 24 h at 37°C. Results are expressed in picograms per milliliter and represent the arithmetic mean ± SEM of 10 experiments. Asterisks represent statistical significance (P < 0.05 vs. controls).
Figure 7.
Acidic values of extracellular pH increase HIV-1 binding to spermatozoa and the subsequent transmission of HIV-1 to DCs. (A) Spermatozoa (Sp; 1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng of p24 for 60 min at 37°C at pH 7.3 (controls), 6.8, 6.5, or 6.0. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The results are expressed as the mean ± SEM of five experiments performed in triplicate. Asterisks represent statistical significance (P < 0.05 vs. pH 7.3). (B) Spermatozoa (1.5 × 106/200 µl) were incubated with different primary HIV-1 isolates containing 25 ng p24 for 60 min at 37°C at pH 7.3 or 6.5. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. A representative experiment performed in duplicate (n = 2–3) is shown. (C) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C at pH 7.3 (controls) or 6.5, in the absence or presence of 10 U/ml heparin. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The results are expressed as the mean ± SEM of four experiments performed in triplicate. *, P < 0.05, heparin vs. controls, at either pH 7.3 or 6.5; **, P < 0.05, controls at pH 6.5 vs. controls at 7.3. (D) Spermatozoa (1.5 × 106/200 µl) were incubated for 60 min at 37°C at pH 7.3 or 6.5, and the expression of HS was then analyzed by flow cytometry. The gray histogram represents isotype control. A representative experiment (n = 3) is shown. (E) 100 µl of whole semen were diluted 1:1 with culture medium, and the pH was adjusted to 7.3 (control) or 6.5. HIV-1 BAL was added containing 25 ng p24, and the samples were incubated for 60 min at 37°C. After centrifugation, cells pellets were washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The results are expressed as the mean ± SEM of four experiments performed in triplicate. The asterisk represents statistical significance (P < 0.05 vs. pH 7.3). (F) DCs (1.5 × 105/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C at pH 7.3 (controls), 6.8, 6.5, or 6.0. Cells were then washed thoroughly, lysed, and assayed for p24 antigen by ELISA. The results are expressed as the mean ± SEM of three experiments performed in triplicate. (G) Spermatozoa (1.5 × 106/200 µl) were incubated with HIV-1 BAL containing 25 ng p24 for 60 min at 37°C, at pH 7.3 or 6.5. Cells were then washed thoroughly and incubated with or without DCs for 7 d at a spermatozoa/DC ratio of 10:1. The infection of DCs was then analyzed by measuring the levels of p24 antigen by ELISA in cell supernatants. The results are expressed as the mean ± SEM of four experiments performed in duplicate. Asterisks represent statistical significance (P < 0.05 vs. spermatozoa cultured without DCs).
Similar articles
- The role of semen in sexual transmission of HIV: beyond a carrier for virus particles.
Sabatté J, Remes Lenicov F, Cabrini M, Rodriguez Rodrigues C, Ostrowski M, Ceballos A, Amigorena S, Geffner J. Sabatté J, et al. Microbes Infect. 2011 Nov;13(12-13):977-82. doi: 10.1016/j.micinf.2011.06.005. Epub 2011 Jul 1. Microbes Infect. 2011. PMID: 21767659 Review. - Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN.
Sabatté J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, Salamone G, Salomón H, Amigorena S, Geffner J. Sabatté J, et al. J Virol. 2007 Dec;81(24):13723-34. doi: 10.1128/JVI.01079-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913809 Free PMC article. - Opposite effects of IL-10 on the ability of dendritic cells and macrophages to replicate primary CXCR4-dependent HIV-1 strains.
Ancuta P, Bakri Y, Chomont N, Hocini H, Gabuzda D, Haeffner-Cavaillon N. Ancuta P, et al. J Immunol. 2001 Mar 15;166(6):4244-53. doi: 10.4049/jimmunol.166.6.4244. J Immunol. 2001. PMID: 11238678 - Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1.
de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. de Witte L, et al. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19464-9. doi: 10.1073/pnas.0703747104. Epub 2007 Nov 26. Proc Natl Acad Sci U S A. 2007. PMID: 18040049 Free PMC article. - Viral piracy: HIV-1 targets dendritic cells for transmission.
Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. Lekkerkerker AN, et al. Curr HIV Res. 2006 Apr;4(2):169-76. doi: 10.2174/157016206776055020. Curr HIV Res. 2006. PMID: 16611055 Review.
Cited by
- CD4-mimetic sulfopeptide conjugates display sub-nanomolar anti-HIV-1 activity and protect macaques against a SHIV162P3 vaginal challenge.
Ariën KK, Baleux F, Desjardins D, Porrot F, Coïc YM, Michiels J, Bouchemal K, Bonnaffé D, Bruel T, Schwartz O, Le Grand R, Vanham G, Dereuddre-Bosquet N, Lortat-Jacob H. Ariën KK, et al. Sci Rep. 2016 Oct 10;6:34829. doi: 10.1038/srep34829. Sci Rep. 2016. PMID: 27721488 Free PMC article. - Vehicles of intercellular communication: exosomes and HIV-1.
Welch JL, Stapleton JT, Okeoma CM. Welch JL, et al. J Gen Virol. 2019 Mar;100(3):350-366. doi: 10.1099/jgv.0.001193. Epub 2019 Jan 31. J Gen Virol. 2019. PMID: 30702421 Free PMC article. Review. - Epigallocatechin-3-gallate local pre-exposure application prevents SHIV rectal infection of macaques.
Liu JB, Li JL, Zhuang K, Liu H, Wang X, Xiao QH, Li XD, Zhou RH, Zhou L, Ma TC, Zhou W, Liu MQ, Ho WZ. Liu JB, et al. Mucosal Immunol. 2018 Jul;11(4):1230-1238. doi: 10.1038/s41385-018-0025-4. Epub 2018 May 31. Mucosal Immunol. 2018. PMID: 29855550 Free PMC article. - Diversity of heparan sulfate and HSV entry: basic understanding and treatment strategies.
Tiwari V, Tarbutton MS, Shukla D. Tiwari V, et al. Molecules. 2015 Feb 5;20(2):2707-27. doi: 10.3390/molecules20022707. Molecules. 2015. PMID: 25665065 Free PMC article. Review. - Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo.
Tiwari V, Liu J, Valyi-Nagy T, Shukla D. Tiwari V, et al. J Biol Chem. 2011 Jul 15;286(28):25406-15. doi: 10.1074/jbc.M110.201103. Epub 2011 May 19. J Biol Chem. 2011. PMID: 21596749 Free PMC article.
References
- Argyris E.G., Acheampong E., Nunnari G., Mukhtar M., Williams K.J., Pomerantz R.J. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140–12151 10.1128/JVI.77.22.12140-12151.2003 - DOI - PMC - PubMed
- Baccetti B., Benedetto A., Burrini A.G., Collodel G., Ceccarini E.C., Crisà N., Di Caro A., Estenoz M., Garbuglia A.R., Massacesi A., et al. 1994. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J. Cell Biol. 127:903–914 10.1083/jcb.127.4.903 - DOI - PMC - PubMed