Engineering lymphocyte subsets: tools, trials and tribulations - PubMed (original) (raw)
Review
Engineering lymphocyte subsets: tools, trials and tribulations
Carl H June et al. Nat Rev Immunol. 2009 Oct.
Abstract
Cell-based therapies with various lymphocyte subsets hold promise for the treatment of several diseases, including cancer and disease resulting from inflammation and infection. The ability to genetically engineer lymphocyte subsets has the potential to improve the natural immune response and correct impaired immunity. In this Review we focus on the lymphocyte subsets that have been modified genetically or by other means for therapeutic benefit, on the technologies used to engineer lymphocytes and on the latest progress and hurdles in translating these technologies to the clinic.
Figures
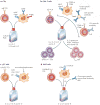
Figure 1. Cell culture approaches for adoptive transfer of lymphocyte subsets
Culture conditions required for distinct lymphocyte subsets vary, depending on the activation and co-stimulatory requirements. a | Cytotoxic T lymphocytes (CTLs) express αβ T cell receptors (TCRs) and are stimulated by antigen-presenting cells (APCs) that express MHC class I molecules. CTLs require 4-1BB ligand (4-1BBL; also known as CD137L) stimulation for clonal expansion and have been expanded in vitro by the addition of feeder cells or artificial APCs that express the co-stimulatory ligands–. b | CD4+ T cells are stimulated by APCs that express peptide-loaded MHC class II complexes. The main co-stimulatory molecule expressed by CD4+ T cells is CD28, which binds to CD80 or CD86 expressed on APCs. Effector CD4+ T cells can be stimulated by artificial APCs (aAPCs) or beads that bear CD3-specific antibody in the presence of various cytokines. Regulatory CD4+ T (TReg) cells require culturing in interleukin-2 (IL-2), and the addition of several reagents to the culture may enhance the suppressive functions of ex vivo expanded TReg cells. c| Cells expressing γδ TCRs, such as the Vγ9Vδ2 TCR expressed by T cells in the blood, are stimulated by APCs that present exogenous isopentenyl pyrophosphate (IPP) or other endogenous ligands stimulated by aminobisphosphonates such as zoledronate,. d| CD1d-restricted Vα24Jα 18+ invaria1nt natural killer T (iNKT) cells are stimulated by α-galactosylceramide (α-GalCer) or the 6B11 clonotype-specific monoclonal antibody. FcR, Fc receptor; HDAC, histone deacetylase.
Similar articles
- Adeno-Associated Viruses (AAV) and Host Immunity - A Race Between the Hare and the Hedgehog.
Rapti K, Grimm D. Rapti K, et al. Front Immunol. 2021 Oct 29;12:753467. doi: 10.3389/fimmu.2021.753467. eCollection 2021. Front Immunol. 2021. PMID: 34777364 Free PMC article. Review. - Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells.
Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Lamers CH, et al. Blood. 2011 Jan 6;117(1):72-82. doi: 10.1182/blood-2010-07-294520. Epub 2010 Oct 1. Blood. 2011. PMID: 20889925 - Adoptive cellular therapy.
Grupp SA, June CH. Grupp SA, et al. Curr Top Microbiol Immunol. 2011;344:149-72. doi: 10.1007/82_2010_94. Curr Top Microbiol Immunol. 2011. PMID: 20700700 Review. - Expression of a human coxsackie/adenovirus receptor transgene permits adenovirus infection of primary lymphocytes.
Schmidt MR, Piekos B, Cabatingan MS, Woodland RT. Schmidt MR, et al. J Immunol. 2000 Oct 1;165(7):4112-9. doi: 10.4049/jimmunol.165.7.4112. J Immunol. 2000. PMID: 11034423 - Genetically modified immune cells targeting tumor antigens.
Poorebrahim M, Abazari MF, Sadeghi S, Mahmoudi R, Kheirollahi A, Askari H, Wickström SL, Poortahmasebi V, Lundqvist A, Kiessling R, Cid-Arregui A. Poorebrahim M, et al. Pharmacol Ther. 2020 Oct;214:107603. doi: 10.1016/j.pharmthera.2020.107603. Epub 2020 Jun 15. Pharmacol Ther. 2020. PMID: 32553789 Review.
Cited by
- Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside.
Zhang B, Yang M, Zhang W, Liu N, Wang D, Jing L, Xu N, Yang N, Ren T. Zhang B, et al. Cell Death Dis. 2024 Jan 15;15(1):50. doi: 10.1038/s41419-024-06438-7. Cell Death Dis. 2024. PMID: 38221520 Free PMC article. Review. - Novel cell and gene therapies for HIV.
Hoxie JA, June CH. Hoxie JA, et al. Cold Spring Harb Perspect Med. 2012 Oct 1;2(10):a007179. doi: 10.1101/cshperspect.a007179. Cold Spring Harb Perspect Med. 2012. PMID: 23028130 Free PMC article. Review. - CAR T cells for infection, autoimmunity and allotransplantation.
Maldini CR, Ellis GI, Riley JL. Maldini CR, et al. Nat Rev Immunol. 2018 Oct;18(10):605-616. doi: 10.1038/s41577-018-0042-2. Nat Rev Immunol. 2018. PMID: 30046149 Free PMC article. Review. - Tales from the Jazz ASH: highlights from the 2013 American Society of Haematology meeting.
Mazzarella L. Mazzarella L. Ecancermedicalscience. 2014 Jan 24;8:390. doi: 10.3332/ecancer.2014.390. eCollection 2014. Ecancermedicalscience. 2014. PMID: 24678345 Free PMC article. - Adoptive immunotherapy for cancer or viruses.
Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Maus MV, et al. Annu Rev Immunol. 2014;32:189-225. doi: 10.1146/annurev-immunol-032713-120136. Epub 2014 Jan 9. Annu Rev Immunol. 2014. PMID: 24423116 Free PMC article. Review.
References
- Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. This was the first study to indicate the potent antitumour effects of human T cells. - PubMed
- Rosenberg SA, et al. Gene transfer into humans — immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. This was the first human gene transfer study. - PubMed
- Uchida N, Cone RD, Freeman GJ, Mulligan RC, Cantor H. High efficiency gene transfer into murine T cell clones using a retroviral vector. J Immunol. 1986;136:1876–1879. - PubMed
- Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. - PubMed
- Amado RG, Chen IS. Lentiviral vectors — the promise of gene therapy within reach? Science. 1999;285:674–676. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA105216/CA/NCI NIH HHS/United States
- P01 AI080192/AI/NIAID NIH HHS/United States
- R01 CA113783/CA/NCI NIH HHS/United States
- R01 AI 34495/AI/NIAID NIH HHS/United States
- 5P01 CA 066726/CA/NCI NIH HHS/United States
- 5R01 CA 105216/CA/NCI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA 72669/CA/NCI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- P30 AI 045008/AI/NIAID NIH HHS/United States
- P01 CA066726/CA/NCI NIH HHS/United States
- 1U19 AI 082628/AI/NIAID NIH HHS/United States
- P01 CA 142106/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- U19 AI066290/AI/NIAID NIH HHS/United States
- U19 AI 066290/AI/NIAID NIH HHS/United States
- P01 AI 056299/AI/NIAID NIH HHS/United States
- U19 AI 082628/AI/NIAID NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- U19 AI082628/AI/NIAID NIH HHS/United States
- R01 AI057838/AI/NIAID NIH HHS/United States
- R41 CA 130547/CA/NCI NIH HHS/United States
- R41 CA130547/CA/NCI NIH HHS/United States
- R01 CA120409/CA/NCI NIH HHS/United States
- 2R01 HL 56067/HL/NHLBI NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- 1R01 CA 120409/CA/NCI NIH HHS/United States
- R01 CA 113783/CA/NCI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- P01 AI 080192/AI/NIAID NIH HHS/United States
- R01 AI 057838/AI/NIAID NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources