Genetic diagnosis by whole exome capture and massively parallel DNA sequencing - PubMed (original) (raw)
. 2009 Nov 10;106(45):19096-101.
doi: 10.1073/pnas.0910672106. Epub 2009 Oct 27.
Ute I Scholl, Weizhen Ji, Tiewen Liu, Irina R Tikhonova, Paul Zumbo, Ahmet Nayir, Ayşin Bakkaloğlu, Seza Ozen, Sami Sanjad, Carol Nelson-Williams, Anita Farhi, Shrikant Mane, Richard P Lifton
Affiliations
- PMID: 19861545
- PMCID: PMC2768590
- DOI: 10.1073/pnas.0910672106
Genetic diagnosis by whole exome capture and massively parallel DNA sequencing
Murim Choi et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Protein coding genes constitute only approximately 1% of the human genome but harbor 85% of the mutations with large effects on disease-related traits. Therefore, efficient strategies for selectively sequencing complete coding regions (i.e., "whole exome") have the potential to contribute to the understanding of rare and common human diseases. Here we report a method for whole-exome sequencing coupling Roche/NimbleGen whole exome arrays to the Illumina DNA sequencing platform. We demonstrate the ability to capture approximately 95% of the targeted coding sequences with high sensitivity and specificity for detection of homozygous and heterozygous variants. We illustrate the utility of this approach by making an unanticipated genetic diagnosis of congenital chloride diarrhea in a patient referred with a suspected diagnosis of Bartter syndrome, a renal salt-wasting disease. The molecular diagnosis was based on the finding of a homozygous missense D652N mutation at a position in SLC26A3 (the known congenital chloride diarrhea locus) that is virtually completely conserved in orthologues and paralogues from invertebrates to humans, and clinical follow-up confirmed the diagnosis. To our knowledge, whole-exome (or genome) sequencing has not previously been used to make a genetic diagnosis. Five additional patients suspected to have Bartter syndrome but who did not have mutations in known genes for this disease had homozygous deleterious mutations in SLC26A3. These results demonstrate the clinical utility of whole-exome sequencing and have implications for disease gene discovery and clinical diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
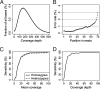
Fig. 1.
Coverage of targeted bases, error rate, and sensitivity to detect variants in whole-exome capture data. (A) Distribution of per-base read coverage among 5 capture experiments. A small fraction of targeted bases are poorly captured across all experiments. (B) The per-base error rate in this data set is shown as a function of read position. (C) Subject GIT 264–1 was sequenced to a mean depth of 99×. The sensitivity to detect homozygous (solid line) or heterozygous (dashed line) variants as mean depth of whole-exome sequence coverage increases from 0 to 100× is shown. Sensitivity to detect heterozygous variants increases from 81% to 90% to 95% as mean coverage is increased from 20× to 30× and 40×, and plateaus at 98%. (D) Sensitivity of detection of heterozygous variants at exact per-base coverage. Sensitivity is approximately 80% at 10× coverage, and approaches 100% at or greater than 20× per-base coverage.
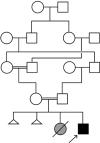
Fig. 2.
Kindred GIT 264. The affected subject is indicated by the arrow, and the pedigree structure demonstrates parental consanguinity. A presumably affected sister died 4 d after premature birth (gray symbol), and there were 2 other spontaneous abortions (small triangles, disease status unknown). The parents and other relatives were free from clinical syndromes like that seen in the index case. For simplification, the mother's 10 siblings and the father's 8 siblings are not depicted in the pedigree.
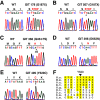
Fig. 3.
Homozygous missense mutation at highly conserved position in SLC26A3 in GIT 264–1. (A) Top: Reference sequence of aa 636–668 of SLC26A3 and the corresponding DNA sequences are shown. Below: Independent Illumina DNA sequence reads from GIT 264–1 are shown. Forward and reverse reads are shown in capital and lowercase letters, respectively. The results demonstrate a homozygous missense mutation, D652N. (B) Sanger sequence of codons 651–653 of SLC26A3 in a WT subject and GIT 264–1 are shown and confirm the D652N mutation. The mutated residue is indicated by an asterisk, and the encoded amino acids are shown in single-letter code. (C) Conservation of D652 across species. The amino acid sequence of segment 648–656 of human SLC26A3 is shown and compared to the corresponding sequence of 6 (of 39) vertebrates and identified in D. melanogaster paralogue and C. elegans orthologue. Positions identical to Homo sapiens (H.s.) are highlighted in yellow. D652 is completely conserved among all species examined. M.m., Mus musculus; O.c., Oryctolagus cuniculus; B.t., Bos taurus; G.g., Gallus gallus; X.l., Xenopus laevis; D.r., Danio rerio; D.m., D. melanogaster; C.e., C. elegans. (D) The sequence of the same segment of human SLC26A3 is compared to 9 paralogues of the human SLC26A gene family (SLC26A1-A11; SLC26A10 is a pseudogene). (E) Structure of SLC26A3. The protein has 12 transmembrane domains and a C-terminal STAS domain (highlighted in blue). The D652N mutation (red circle) lies in the STAS domain.
Fig. 4.
Additional patients with SLC26A3 mutations. Sanger sequence traces of WT and 5 subjects with homozygous mutations in SLC26A3 are shown. These include two subjects with premature termination at codon 187 (A and B); a frameshift at codon 454 resulting from a single base deletion that leads to termination at codon 458 (C); a second patient with the D652N mutation (D); and a patient with a Y520C mutation (E). (F) Conservation of Y520 across species: Y520 is conserved among all orthologues and paralogues examined.
Similar articles
- Mimicry and well known genetic friends: molecular diagnosis in an Iranian cohort of suspected Bartter syndrome and proposition of an algorithm for clinical differential diagnosis.
Najafi M, Kordi-Tamandani DM, Behjati F, Sadeghi-Bojd S, Bakey Z, Karimiani EG, Schüle I, Azarfar A, Schmidts M. Najafi M, et al. Orphanet J Rare Dis. 2019 Feb 13;14(1):41. doi: 10.1186/s13023-018-0981-5. Orphanet J Rare Dis. 2019. PMID: 30760291 Free PMC article. - Comprehensive comparison of three commercial human whole-exome capture platforms.
Asan, Xu Y, Jiang H, Tyler-Smith C, Xue Y, Jiang T, Wang J, Wu M, Liu X, Tian G, Wang J, Wang J, Yang H, Zhang X. Asan, et al. Genome Biol. 2011 Sep 28;12(9):R95. doi: 10.1186/gb-2011-12-9-r95. Genome Biol. 2011. PMID: 21955857 Free PMC article. - Significance of molecular testing for congenital chloride diarrhea.
Lechner S, Ruemmele FM, Zankl A, Lausch E, Huber WD, Mihatsch W, Phillips AD, Lewindon P, Querfeld U, Heinz-Erian P, Müller T, Janecke AR. Lechner S, et al. J Pediatr Gastroenterol Nutr. 2011 Jul;53(1):48-54. doi: 10.1097/MPG.0b013e31820bc856. J Pediatr Gastroenterol Nutr. 2011. PMID: 21694535 - Congenital chloride diarrhea in patient with SLC26A2 mutation - analysis of the clinical phenotype and differential diagnosis.
Sun M, Tao N, Liu X, Yang Y, Su Y, Xu F. Sun M, et al. Pediatr Endocrinol Diabetes Metab. 2021;27(1):51-56. doi: 10.5114/pedm.2020.97465. Pediatr Endocrinol Diabetes Metab. 2021. PMID: 33599438 Free PMC article. Review. - Exome and genome sequencing: a revolution for the discovery and diagnosis of monogenic disorders.
Stranneheim H, Wedell A. Stranneheim H, et al. J Intern Med. 2016 Jan;279(1):3-15. doi: 10.1111/joim.12399. Epub 2015 Aug 7. J Intern Med. 2016. PMID: 26250718 Review.
Cited by
- Comparative analysis of whole exome sequencing kits for the canine genome.
Jang J, Lee YJ, Ko S, Abd El-Aty AM, Gecili I, Jeong JH, Kwon C, Jung TW. Jang J, et al. PLoS One. 2024 Nov 4;19(11):e0312203. doi: 10.1371/journal.pone.0312203. eCollection 2024. PLoS One. 2024. PMID: 39495750 Free PMC article. - The application of whole-exome sequencing in the early diagnosis of rare genetic diseases in children: a study from Southeastern China.
Lai G, Gu Q, Lai Z, Chen H, Chen J, Huang J. Lai G, et al. Front Pediatr. 2024 Oct 8;12:1448895. doi: 10.3389/fped.2024.1448895. eCollection 2024. Front Pediatr. 2024. PMID: 39439447 Free PMC article. - A Comprehensive Approach to the Diagnosis of Leigh Syndrome Spectrum.
Baldo MS, Azevedo L, Coelho MP, Martins E, Vilarinho L. Baldo MS, et al. Diagnostics (Basel). 2024 Sep 25;14(19):2133. doi: 10.3390/diagnostics14192133. Diagnostics (Basel). 2024. PMID: 39410537 Free PMC article. - Whole-exome sequencing reveals Kawasaki disease susceptibility genes and their association with coronary artery lesion.
Wang Y, Chen X, Zhang D, Chen R, Alifu A. Wang Y, et al. Front Pediatr. 2024 Sep 10;12:1400123. doi: 10.3389/fped.2024.1400123. eCollection 2024. Front Pediatr. 2024. PMID: 39318622 Free PMC article.
References
- Cooper DN, Krawczak M, Antonorakis SE. The nature and mechanisms of human gene mutation. In: Scriver C, Beaudet al., Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. New York: McGraw-Hill; 1995. pp. 259–291.
- Hirschhorn JN. Genomewide association studies−illuminating biologic pathways. N Engl J Med. 2009;360:1699–1701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases