Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction - PubMed (original) (raw)
Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction
Benjamin Lovegren de Bivort et al. J Neurogenet. 2009.
Abstract
The cell-surface-signaling protein Notch, is required for numerous developmental processes and typically specifies which of two adjacent cells will adopt a non-neuronal developmental fate. It has recently been implicated in long-term memory formation in mammals and Drosophila. Here, we investigated whether activity-dependent synaptic plasticity at the neuromuscular junctions (NMJs) of third instar Drosophila larvae depends on Notch signaling. The length and number of axonal branches and number of presynaptic sites (boutons) in NMJ vary with the level of synaptic activity, so we increased activity at the NMJ by two complementary methods: increasing the chronic growth temperature of third instar larvae from 18 to 28 degrees C and using the double-mutant ether-a-gogo,Shaker (eagSh), both of which increase NMJ size and bouton count. Animals homozygous for the functionally null, temperature-sensitive Notch alleles, N(ts1) and N(ts2), displayed no activity-dependent increase in NMJ complexity when reared at the restrictive temperature. Dominant-negative Notch transgenic expression also blocked activity-dependent plasticity. Ectopic expression of wild-type Notch and constitutively active truncated Notch transgenes also reduced activity-dependent plasticity, suggesting that there is a "happy medium" level of Notch activity in mediating NMJ outgrowth. Last, we show that endogenous Notch is primarily expressed in the presynaptic cell bodies where its expression level is positively correlated with motor neuron activity.
Figures
Figure 1. Notch mutant alleles block temperature-induced NMJ outgrowth
A) Representative camera lucida examples of NMJ arbors from Nts1 and Nts2 alleles, muscle fibers 7 and 6, 13 and 12, and low and high temperatures. B) Quantification of NMJ complexity is shown as the average number of boutons and branches per arbor under the above experimental conditions. Bars in the top half represent the mean number of boutons, and in the bottom half, the number of branches. Error bars are +/− standard error of the mean. Horizontal lines at 100 boutons and 10 branches have been added for visual reference. P-values are given for the comparison between the adjacent 18°C and 29°C categories. For all figures, *** = p-value < 0.005, ** = 0.005 < p-value < 0.01, * = 0.01 < p-value < 0.05. The number of replicates per experiment is between 9 and 19 for all experiments in all figures unless otherwise noted. Bouton counts were not normalized for muscle fiber area, as the latter did not vary with statistical significance across experimental groups.
Figure 2. Representative Branching Diagrams
Nine muscle fiber 12 NMJ branching diagrams from each category were chosen at random to give a representative sample of the branching diversity and patterns for each experimental group. Nts1 arbors have the same topological distribution at 18°C and 29°C as the wild type arbors at 18°C. Wild type arbors at 29°C have many more branches and sub-branches.
Figure 3. hs-NΔcdc10rpts dominant negative transgene blocks temperature-induced NMJ outgrowth
A) Expression of the temperature-inducible Notch dominant negative transgene NΔcdc10rpts blocks temperature-dependent increase in bouton and branch counts on muscle fibers 13 and 12. P-value asterisks compare wild type to NΔcdc10rpts NMJs in equivalent treatments. B) Mild heat shocks generally do not reduce NMJ complexity in NΔcdc10rpts animals incubated primarily at 18°C, which do not appear statistically different from wild type when reared entirely at 18°C. One hour heat shocks at 29°C and 37°C per day are sufficient to reduce NMJ branching on muscle fiber 12 by ~27%. P-value asterisks compare each heat-shock condition to constant 18°C incubation.
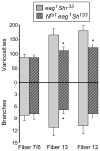
Figure 4. Notch inactivation blocks NMJ plasticity induced by the activity double mutant eagSh
P-value asterisks compare Notchts1,eag,Sh and eag,Sh for each muscle fiber at room temperature. Notchts1,eag,Sh triple mutant lines grew weakly, and n=6 for each of these conditions. P-value asterisks compare high temperature to low temperature for each muscle fiber.
Figure 5. hs-N and hs-Nintra ectopic Notch expression transgenes reduce temperature-induced NMJ outgrowth
Bouton counts do not increase at high temperatures in lines ectopically expressing Notch (hs-N) or constitutively active Notch truncation hs-Nintra. All experimental lines were grown chronically at 29°C, inducing both temperature-dependent plasticity as well as the expression of the _hs_-dependent transgenes. P-value asterisks compare high temperature to low temperature for each genotype.
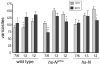
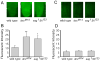
Figure 6. Notch expression levels vary with neural activity
A) Representative FITC confocal images of the subesophageal ganglion (motor ganglion) of the third instar larval brain, stained with antibodies against the NECD. Notch expression is higher in dnc and eag,Sh mutants which also increase neural activity. B) Average fluorescent intensity of subesophageal ganglion across 10 replicates of each genotype, quantified from the confocal images. P-value asterisks compare each genotype to wild type. C) Confocal images of Notch expression in the muscle fibers of the 4th abdominal segment. D) Quantified fluorescence in the muscle fibers. No significant variation was observed here in the mutants with higher activity. Vertical axes in B and D are in arbitrary units of fluorescent intensity with 256 as a sensor maximum.
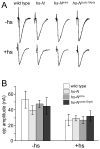
Figure 7. NMJ electrophysiological responses are the same in Notch mutants and wild type
A) Typical EJCs recorded in muscle fiber 12 following stimulation of the incoming nerve. −hs animals were reared entirely at room temperature, +hs animals were incubated at 37°C for 1 hour per day, starting from the first instar. Heat shock caused a reduction in EJC magnitude that was independent of both Notch ectopic activation and inhibition. B) Quantification of EJC magnitudes. No statistically significant differences were seen between fly lines at fixed temperature conditions. 4 < n < 6 for all experiments.
Similar articles
- The hangover gene negatively regulates bouton addition at the Drosophila neuromuscular junction.
Schwenkert I, Eltrop R, Funk N, Steinert JR, Schuster CM, Scholz H. Schwenkert I, et al. Mech Dev. 2008 Aug;125(8):700-11. doi: 10.1016/j.mod.2008.04.004. Epub 2008 Apr 27. Mech Dev. 2008. PMID: 18524547 - Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function.
Xing B, Ashleigh Long A, Harrison DA, Cooper RL. Xing B, et al. Synapse. 2005 Sep 1;57(3):132-47. doi: 10.1002/syn.20165. Synapse. 2005. PMID: 15945059 - Structural and Functional Synaptic Plasticity Induced by Convergent Synapse Loss in the Drosophila Neuromuscular Circuit.
Wang Y, Lobb-Rabe M, Ashley J, Anand V, Carrillo RA. Wang Y, et al. J Neurosci. 2021 Feb 17;41(7):1401-1417. doi: 10.1523/JNEUROSCI.1492-20.2020. Epub 2021 Jan 5. J Neurosci. 2021. PMID: 33402422 Free PMC article. - Development and plasticity of the Drosophila larval neuromuscular junction.
Menon KP, Carrillo RA, Zinn K. Menon KP, et al. Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):647-70. doi: 10.1002/wdev.108. Epub 2013 Feb 5. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014452 Free PMC article. Review. - Regulation of notch signaling via O-glucosylation insights from Drosophila studies.
Lee TV, Takeuchi H, Jafar-Nejad H. Lee TV, et al. Methods Enzymol. 2010;480:375-98. doi: 10.1016/S0076-6879(10)80017-5. Methods Enzymol. 2010. PMID: 20816218 Review.
Cited by
- Notch signaling pathway is activated in motoneurons of spinal muscular atrophy.
Caraballo-Miralles V, Cardona-Rossinyol A, Garcera A, Torres-Benito L, Soler RM, Tabares L, Lladó J, Olmos G. Caraballo-Miralles V, et al. Int J Mol Sci. 2013 May 29;14(6):11424-37. doi: 10.3390/ijms140611424. Int J Mol Sci. 2013. PMID: 23759991 Free PMC article. - Mind Bomb-2 Regulates Hippocampus-dependent Memory Formation and Synaptic Plasticity.
Kim S, Kim T, Lee HR, Kong YY, Kaang BK. Kim S, et al. Korean J Physiol Pharmacol. 2015 Nov;19(6):515-22. doi: 10.4196/kjpp.2015.19.6.515. Epub 2015 Oct 16. Korean J Physiol Pharmacol. 2015. PMID: 26557018 Free PMC article. - Regulation of striatal dopamine responsiveness by Notch/RBP-J signaling.
Toritsuka M, Kimoto S, Muraki K, Kitagawa M, Kishimoto T, Sawa A, Tanigaki K. Toritsuka M, et al. Transl Psychiatry. 2017 Mar 7;7(3):e1049. doi: 10.1038/tp.2017.21. Transl Psychiatry. 2017. PMID: 28267151 Free PMC article. - LIN-12/Notch signaling instructs postsynaptic muscle arm development by regulating UNC-40/DCC and MADD-2 in Caenorhabditis elegans.
Li P, Collins KM, Koelle MR, Shen K. Li P, et al. Elife. 2013 Mar 19;2:e00378. doi: 10.7554/eLife.00378. Elife. 2013. PMID: 23539368 Free PMC article. - Alcohol Activates Scabrous-Notch to Influence Associated Memories.
Petruccelli E, Feyder M, Ledru N, Jaques Y, Anderson E, Kaun KR. Petruccelli E, et al. Neuron. 2018 Dec 5;100(5):1209-1223.e4. doi: 10.1016/j.neuron.2018.10.005. Epub 2018 Oct 25. Neuron. 2018. PMID: 30482693 Free PMC article.
References
- Alton AK, Fechtel K, Kopczynski CC, Shepard SB, Kooh PJ, Muskavitch MA. Molecular genetics of Delta, a locus required for ectodermal differentiation in Drosophila. Dev Genet. 1989;10:261–272. - PubMed
- Berezovska O, McLean P, Knowles R, Frosh M, Lu FM, Lux SE, Hyman BT. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases