The E3 ubiquitin ligases Hrd1 and gp78 bind to and promote cholera toxin retro-translocation - PubMed (original) (raw)
The E3 ubiquitin ligases Hrd1 and gp78 bind to and promote cholera toxin retro-translocation
Kaleena M Bernardi et al. Mol Biol Cell. 2010.
Abstract
To cause disease, cholera toxin (CT) is transported from the cell surface to the endoplasmic reticulum (ER) lumen where the catalytic CTA1 subunit retro-translocates to the cytosol to induce pathological water secretion. Two retro-translocon components are the Derlins and ER-associated multi-spanning E3 ubiquitin ligases including Hrd1 and gp78. We demonstrated previously that Derlin-1 facilitates CTA1 retro-translocation. However, as CTA1 is neither ubiquitinated on lysines nor at its N-terminus, the role of E3 ligases in toxin retro-translocation is unclear. Here, we show that expression of mutant Hrd1 and gp78 and a mutant E2-conjugating enzyme dedicated to retro-translocation (Ube2g2) decrease CTA1 retro-translocation. Hrd1 knockdown also attenuated toxin retro-translocation. Binding studies demonstrate that Hrd1 and gp78 interact with CT and protein disulfide isomerase, an ER chaperone that unfolds CTA1 to initiate translocation. Moreover, we find that the toxin's association with Hrd1 and gp78 is blocked by dominant-negative Derlin-1, suggesting that CT is targeted initially to Derlin-1 and then transferred to Hrd1 and gp78. These data demonstrate a role of the E3 ubiquitin ligases in CTA1 retro-translocation, implicate a sequence of events experienced by the toxin on the ER membrane, and raise the possibility that ubiquitination is involved in the transport process.
Figures
Figure 1.
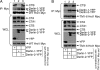
Hrd1 facilitates retro-translocation of CTA1. (A) Lysates from 293T cells expressing YFP, WT Hrd1 Myc, C291A Hrd1 Myc, TM1-6 Hrd1 Myc, or cyt Hrd1 Myc were analyzed for expression of Hrd1 proteins and the ER stress markers BiP and Derlin-1. (B) RT-PCR analysis of the unspliced (u) and spliced (s) forms of the XBP1 mRNA from cells treated with DTT or tunicamycin or from cells expressing YFP, WT Hrd1 Myc, C291A Hrd1 Myc, or TM1-6 Hrd1 Myc. (C) Membrane association of TM1-6 Hrd1 Myc. Cells expressing TM1-6 Hrd1 Myc were subjected to alkali extraction and pelleted, and the supernatant and pellet fractions were separated. Samples were immunoblotted with an Myc antibody, a PDI antibody (soluble marker), and a Derlin-1 antibody (membrane marker). (D and E) 293T cells expressing the indicated combinations of WT Hrd1 FLAG, ERp29 FLAG, WT Hrd1 Myc, C291A Hrd1 Myc, TM1-6 Hrd1 Myc, and cyt Hrd1 Myc were harvested and lysed in a 1% Triton X-100 buffer. Lysates were subjected to immunoprecipitation with the indicated antibodies. The immunoprecipitation complexes were analyzed by SDS-PAGE and immunoblotted with polyclonal antibodies to either FLAG or Myc. (F) Cells expressing YFP, WT Hrd1 Myc, C291A Hrd1 Myc, or TM1-6 Hrd1 Myc were treated with 10 nM CT and subjected to the retro-translocation assay. Supernatant and pellet fractions were analyzed by nonreducing SDS-PAGE, followed by immunoblotting with indicated antibodies. CTA is 28 kDa and CTA1 is 22 kDa. (G) The intensity of the CTA1 band generated in E was quantified with ImageJ (
). Mean of five independent experiments; error bars, ± SD. (H) 293T cells expressing YFP, WT Hrd1 Myc, C291A Hrd1 Myc, or TM1-6 Hrd1 Myc were incubated with CT for 90 min, and the cAMP level was measured with a cAMP Biotrak Enzyme Immunoassay System (GE Healthcare, Waukesha, WI). Data were normalized against the forskolin-induced cAMP level, as demonstrated previously (Forster et al., 2006). (I) Cells transfected with a scrambled or a Hrd1-specific siRNA were either lysed and the lysates immunoblotted with the indicated antibodies or were subjected to the retro-translocation assay as described in F. Quantification of the CTA1 band intensity as in G is shown below.
Figure 2.
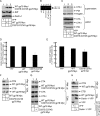
Hrd1 binds to CTB and CTA. (A) 293T cells expressing YFP, WT Hrd1 Myc, C291A Hrd1 Myc, or TM1-6 Hrd1 Myc were treated with 10 nM CT for 90 min. Cells were harvested and lysed in 1% Triton X-100 buffer. Lysates were subjected to immunoprecipitation with a monoclonal Myc antibody. The precipitated complexes were subjected to nonreducing SDS-PAGE and immunoblotted with the indicated antibodies. WCL, whole cell lysate. (B) As in A, except in HeLa cells. (C) As in A, except cells were treated with 100 nM CT and lysed in a 1% deoxyBigChap buffer. Derlin-1-YFP was precipitated with a GFP antibody. The CTA band intensity was quantified as in Figure 1. (D) Endogenous Hrd1 was immunoprecipitated from cells intoxicated with or without CT using either a control Myc or a Hrd1-specific antibody, and the immunoprecipitate was analyzed as in A.
Figure 3.
Expression of Derlin-1-YFP blocks the interaction between WT Hrd1/TM1-6 Hrd1 and CTB. (A) 293T cells expressing WT Hrd1 Myc and either YFP, Derlin-1-YFP or Derlin-2-YFP were treated with 10 nM CT for 90 min, harvested, and lysed in a 1% Triton X-100 buffer. Lysates were subjected to immunoprecipitation with monoclonal Myc antibody. Precipitated complexes were analyzed by nonreducing SDS-PAGE and immunoblotted with the indicated antibodies. (B) As in A, except TM1-6 Hrd1 Myc was expressed.
Figure 4.
Expression of a catalytic-inactive gp78 mutant decreases CTA1 retro-translocation. (A) 293T cells expressing YFP, WT gp78 Myc, or C337S:C374S gp78 Myc were harvested and lysed, and the lysates were subjected to SDS-PAGE and analyzed with the indicated antibodies. (B–E) As in Figure 1, except gp78 constructs were used. (F and G) As in Figure 2, except gp78 constructs were used. Asterisk denotes unidentified protein. (H) As in Figure 3, except WT gp78 Myc was cotransfected with either YFP or Derlin-1-YFP.
Figure 5.
Expression of Ube2g2 mutants decreases CTA1 retro-translocation. (A) Purified recombinant WT Ube2g2 (400 nM), Ube2g2 delta loop (400 nM), and Ube2g2 H94K (400 nM) were incubated with or without the cytosolic domain of gp78 (gp78c, 200 or 400 nM) for 12 min at 37°C. The samples were subjected to nonreducing SDS-PAGE and immunoblotted with an Ube2g2 antibody. E2∼Ub, one ubiquitin molecule anchored to E2. E2∼Ub(n), multiple ubiquitin molecules anchored to E2. (B) 293T cells expressing YFP, WT FLAG Ube2g2, FLAG Ube2g2delta loop, or FLAG Ube2g2H94K were harvested and lysed, and the lysates were subjected to immunoblot analysis with the indicated antibodies. (C) 293T cells expressing YFP, WT FLAG Ube2g2, FLAG Ube2g2delta loop, or FLAG Ube2g2H94K were treated with 10 nM CT for 90 min and subjected to the retro-translocation assay described in Figure 1. The supernatant fraction is shown. (D) The intensity of the CTA1 band in the supernatant fraction was quantified with ImageJ. Mean of three independent experiments; error bars, ± SD.
Figure 6.
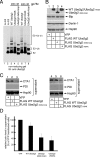
Hrd1 and gp78 bind to PDI. (A) 293T cells were transfected with WT PDI FLAG (lane 1) or cotransfected with WT PDI FLAG and WT Hrd1 Myc (lane 2). In lanes 3–4, cells were transfected with WT Hrd1 Myc and either WT PDI FLAG or I272W FLAG. Cells were harvested, treated with DSP for 30 min, and then lysed in a 1% Triton X-100 buffer. Lysates were subjected to immunoprecipitation with a monoclonal Myc antibody. Complexes were subjected to reducing SDS-PAGE and immunoblotted with the indicated antibodies. WCL, whole cell lysate. (B) As in A, except WT gp78 Myc was expressed.
Similar articles
- The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin.
Moore P, Bernardi KM, Tsai B. Moore P, et al. Mol Biol Cell. 2010 Apr 1;21(7):1305-13. doi: 10.1091/mbc.e09-09-0826. Epub 2010 Feb 3. Mol Biol Cell. 2010. PMID: 20130085 Free PMC article. - Derlin-1 facilitates the retro-translocation of cholera toxin.
Bernardi KM, Forster ML, Lencer WI, Tsai B. Bernardi KM, et al. Mol Biol Cell. 2008 Mar;19(3):877-84. doi: 10.1091/mbc.e07-08-0755. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094046 Free PMC article. - The ERdj5-Sel1L complex facilitates cholera toxin retrotranslocation.
Williams JM, Inoue T, Banks L, Tsai B. Williams JM, et al. Mol Biol Cell. 2013 Mar;24(6):785-95. doi: 10.1091/mbc.E12-07-0522. Epub 2013 Jan 30. Mol Biol Cell. 2013. PMID: 23363602 Free PMC article. - Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum.
Wernick NL, Chinnapen DJ, Cho JA, Lencer WI. Wernick NL, et al. Toxins (Basel). 2010 Mar;2(3):310-25. doi: 10.3390/toxins2030310. Epub 2010 Mar 5. Toxins (Basel). 2010. PMID: 22069586 Free PMC article. Review. - [Physiological Roles of Ubiquitin Ligases Related to the Endoplasmic Reticulum].
Kaneko M. Kaneko M. Yakugaku Zasshi. 2016;136(6):805-9. doi: 10.1248/yakushi.15-00292-2. Yakugaku Zasshi. 2016. PMID: 27252059 Review. Japanese.
Cited by
- A small-molecule inhibitor and degrader of the RNF5 ubiquitin ligase.
Ruan J, Liang D, Yan W, Zhong Y, Talley DC, Rai G, Tao D, LeClair CA, Simeonov A, Zhang Y, Chen F, Quinney NL, Boyles SE, Cholon DM, Gentzsch M, Henderson MJ, Xue F, Fang S. Ruan J, et al. Mol Biol Cell. 2022 Nov 1;33(13):ar120. doi: 10.1091/mbc.E22-06-0233. Epub 2022 Sep 8. Mol Biol Cell. 2022. PMID: 36074076 Free PMC article. - Requirements for mouse mammary tumor virus Rem signal peptide processing and function.
Byun H, Halani N, Gou Y, Nash AK, Lozano MM, Dudley JP. Byun H, et al. J Virol. 2012 Jan;86(1):214-25. doi: 10.1128/JVI.06197-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072771 Free PMC article. - The Ero1alpha-PDI redox cycle regulates retro-translocation of cholera toxin.
Moore P, Bernardi KM, Tsai B. Moore P, et al. Mol Biol Cell. 2010 Apr 1;21(7):1305-13. doi: 10.1091/mbc.e09-09-0826. Epub 2010 Feb 3. Mol Biol Cell. 2010. PMID: 20130085 Free PMC article. - Toxins Utilize the Endoplasmic Reticulum-Associated Protein Degradation Pathway in Their Intoxication Process.
Nowakowska-Gołacka J, Sominka H, Sowa-Rogozińska N, Słomińska-Wojewódzka M. Nowakowska-Gołacka J, et al. Int J Mol Sci. 2019 Mar 15;20(6):1307. doi: 10.3390/ijms20061307. Int J Mol Sci. 2019. PMID: 30875878 Free PMC article. Review. - The Gp78 ubiquitin ligase: probing endoplasmic reticulum complexity.
St Pierre P, Nabi IR. St Pierre P, et al. Protoplasma. 2012 Feb;249 Suppl 1:S11-8. doi: 10.1007/s00709-011-0344-8. Epub 2011 Nov 3. Protoplasma. 2012. PMID: 22045301 Review.
References
- Cadwell K., Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. - PubMed
- Dixit G., Mikoryak C., Hayslett T., Bhat A., Draper R. K. Cholera toxin up-regulates endoplasmic reticulum proteins that correlate with sensitivity to the toxin. Exp. Biol. Med. 2008;233:163–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources