CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria - PubMed (original) (raw)
CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria
Laura K Erdman et al. J Immunol. 2009.
Abstract
CD36 participates in macrophage internalization of a variety of particles, and has been implicated in inflammatory responses to many of these ligands. To what extent CD36 cooperates with other receptors in mediating these processes remains unclear. Because CD36 has been shown to cooperate with TLR2, we investigated the roles and interactions of CD36 and TLRs in inflammation and phagocytosis. Using Ab-induced endocytosis of CD36 and phagocytosis of erythrocytes displaying Abs to CD36, we show that selective engagement and internalization of this receptor did not lead to proinflammatory cytokine production by primary human and murine macrophages. In addition, CD36-mediated phagocytosis of Plasmodium falciparum malaria-parasitized erythrocytes (PEs), which contain parasite components that activate TLRs, also failed to induce cytokine secretion from primary macrophages. Furthermore, we demonstrate that CD36-mediated internalization did not require TLR2 or the TLR-signaling molecule IRAK4. However, macrophage pretreatment with TLR agonists markedly stimulated particle uptake via CD36. Similarly, PE uptake was unaffected by TLR deficiency, but in wild-type cells was increased by pretreatment with purified P. falciparum glycosylphosphatidylinositols, which activate TLR2. Our findings indicate that CD36 must cooperate with other receptors such as TLRs to participate in cytokine responses. Although purified P. falciparum components activate TLRs, CD36-mediated internalization of intact PEs is not inflammatory. Further, CD36 mediates internalization of particles, including PEs, independently of TLR signaling, but can functionally cooperate with TLRs to enhance internalization.
Figures
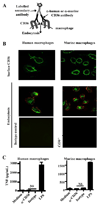
Fig. 1. Antibody-induced CD36 cross-linking and endocytosis do not stimulate secretion of pro-inflammatory cytokines
(A) Diagrammatic representation of antibody-induced CD36 cross-linking and endocytosis model. (B) Upper panels: Surface CD36 was cross-linked on primary human and murine macrophages by incubation at 4°C with α-CD36 antibody, followed by a FITC- or Cy2-conjugated secondary antibody. Middle panels: Following transfer of macrophages to 37°C, CD36-antibody complexes were internalized. Internalization of the complexes was confirmed by labeling the remaining surface CD36 with a tertiary Cy3-coupled antibody and examining vertical sections of cells constructed from Z-stacks (above and to left of individual images). Green signal within the macrophage represents internalized CD36-antibody complexes. Lower panels: The CD36-specific nature of the system was demonstrated by treating human macrophages with an isotype-matched antibody (left) and using _Cd36_−/− murine macrophages (right). (C) Macrophages were incubated at 37°C for 24 hr following CD36 crosslinking, and TNF levels in supernatants were assessed by ELISA. ND, not detectable. NS, not significant by Student’s t-test. Results are representative of three independent experiments.
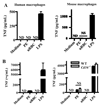
Fig. 2. CD36-mediated phagocytosis of large particles does not result in secretion of proinflammatory cytokines
(A) Schematic diagram of human and mouse α-CD36 EBABs. α-CD36 antibodies conjugated to human red blood cells (RBC) via biotin and streptavidin crosslink CD36 on the macrophage surface, resulting in internalization. (B) Differential interference contrast images of α-CD36 EBABs binding to wild-type and _Cd36_−/− murine macrophages (upper panels) and internalization by wild-type macrophages (lower panels). After an internalization assay, EBAB uptake was confirmed by staining bound EBABs with a fluorophore-conjugated antibody; following a hypotonic lysis, the ghosts of lysed bound EBABs appeared stained, whereas internalized EBABs (indicated by arrows) did not. (C) Human macrophages (top) and wild-type and _Cd36_−/− murine macrophages (bottom) were incubated with α-CD36 EBABs and EBABs prepared with an isotype-matched control antibody, and phagocytosis was assessed. ** indicates p<0.01 by Student’s t-test (human data) or one-way ANOVA with Bonferroni post-tests (murine data). (D) α-CD36 EBABs or isotype control EBABs were incubated with human (top) or murine (bottom) macrophages at 37°C for 4 and 8 hr, and TNF in supernatants was measured by ELISA. NS, not significant by Student’s t-test. Results are representative of three independent experiments.
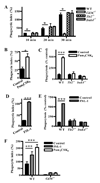
Fig. 3. CD36-mediated internalization of P. falciparum PEs by macrophages does not stimulate pro-inflammatory cytokine secretion
(A) Human macrophages (left) and wild-type murine macrophages (right) were incubated for 4 hr at 37°C with carefully synchronized and washed mature-stage PEs, uninfected red blood cells (uRBC), or LPS. Supernatants were collected and analyzed by ELISA for TNF. NS, not significant by Student’s t-test. (B) To ensure that lack of priming was not obscuring inflammatory consequences of CD36-mediated PE internalization, macrophages were pre-incubated for 12 hr with IFN-γ (100 U/mL) prior to the internalization assay. _Cd36_−/− murine macrophages were included as a control to assess the contribution of CD36-mediated PE internalization to the low levels of TNF production observed. NS, not significant by Student’s t-test (human data); *** indicates p<0.001 and NS indicates not significant by one-way ANOVA with Bonferroni post-tests (murine data). ND, not detectable. Results are representative of three independent experiments.
Fig. 4. Macrophage pre-stimulation by TLR2 agonists enhances CD36-mediated internalization in a TLR2-, IRAK4-, and CD36-dependent manner
(A) Wild-type, _Tlr2_−/−, _Irak4_−/−, and _Cd36_−/− murine macrophages were incubated with α-CD36 EBABs over a 30 min time course, and phagocytic indices were determined. * indicates p<0.05 by one-way ANOVA with Bonferroni post-tests. (B) Human and (C) murine macrophages were treated with medium alone or TLR2 agonist Pam3CSK4 (100 ng/mL) for 1 hr, followed by a 30 min α-CD36 EBAB internalization assay. (D) Human and (E) murine macrophages were treated with medium alone or CD36-dependent TLR2 agonist FSL-1 (20 ng/mL) for 1 hour prior to α-CD36 EBAB internalization. (F) Wild-type and _Cd36_−/− murine macrophages were pre-stimulated with medium alone, Pam3CSK4, or FSL-1 for 1 hr prior to α-CD36 EBAB internalization. * indicates p<0.05, ** p<0.01, and *** p<0.001 by Student’s t-test (human data) or two-way ANOVA with Bonferroni post-tests (murine data). Data shown are representative of three independent experiments.
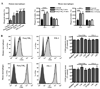
Fig. 5. Enhancement of CD36-mediated internalization by TLR2 stimulation occurs with brief pre-treatment periods and is not related to increased surface levels of CD36
(A) Pre-stimulation with FSL-1 and Pam3CSK4 for 5 min and 15 min significantly increased uptake of α-CD36 EBABs by human macrophages (left) and wild-type murine macrophages, but not _Tlr2_−/− macrophages (right). * indicates p<0.05, ** p<0.01, and *** p<0.001 by one-way ANOVA (human data) or two-way ANOVA with Bonferroni post-tests (murine data). (B) Human and wild-type murine macrophages were incubated with medium alone (light gray shaded histogram) or with FSL-1 or Pam3CSK4 for 15 min (thick black line), 1 hr (gray line), or 2hr (thin black line). Cells were stained for surface CD36 and analyzed by flow cytometry. The dark gray shaded histogram indicates macrophages incubated with the appropriate isotype-matched antibody control. Histogram plots (left) show data from a representative experiment. For at least three independent experiments, the geometric means of CD36 levels were normalized to medium alone and pooled (right). None of the treatments induced a significant change in CD36 levels as analyzed by one-way ANOVA.
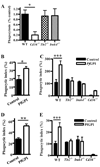
Fig. 6. TLRs are not required for CD36-mediated PE internalization, but macrophage pre-stimulation with a malaria TLR2 agonist enhances internalization of α-CD36 EBABs and PEs
(A) Wild-type, _Tlr2_−/−, _Irak4_−/−, and _Cd36_−/− murine macrophages were incubated with P. falciparum PEs for 2 hr. Data represent the results of at least 5 independent experiments expressed as a percentage of the phagocytic index of wild-type macrophages and pooled together. * indicates p<0.05 by one-way ANOVA with Bonferroni post-tests. (B) Human and (C) murine macrophages were pre-treated with gold beads alone (control) or HPLC-purified _Pf_GPI (400 ng/mL) for 1 hr, followed by a 30 min α-CD36 EBAB internalization assay. (D) Human and (E) murine macrophages were pre-treated with _Pf_GPI or control beads for 2 hr, followed by 2 hr PE internalization assay. * indicates p<0.05, ** p<0.01, and *** p<0.001 by Student’s t-test (human data) or two-way ANOVA with Bonferroni post-tests (murine data). Results are representative of at least two independent experiments.
Similar articles
- CD36 modulates proinflammatory cytokine responses to Plasmodium falciparum glycosylphosphatidylinositols and merozoites by dendritic cells.
Kumar S, Gowda NM, Wu X, Gowda RN, Gowda DC. Kumar S, et al. Parasite Immunol. 2012 Jul;34(7):372-82. doi: 10.1111/j.1365-3024.2012.01367.x. Parasite Immunol. 2012. PMID: 22486596 Free PMC article. - The Toll-like receptor 2 (TLR2) ligand FSL-1 is internalized via the clathrin-dependent endocytic pathway triggered by CD14 and CD36 but not by TLR2.
Shamsul HM, Hasebe A, Iyori M, Ohtani M, Kiura K, Zhang D, Totsuka Y, Shibata K. Shamsul HM, et al. Immunology. 2010 Jun;130(2):262-72. doi: 10.1111/j.1365-2567.2009.03232.x. Epub 2010 Jan 27. Immunology. 2010. PMID: 20113368 Free PMC article. - Regulation of immune response by Plasmodium-infected red blood cells.
Ndungu FM, Urban BC, Marsh K, Langhorne J. Ndungu FM, et al. Parasite Immunol. 2005 Oct-Nov;27(10-11):373-84. doi: 10.1111/j.1365-3024.2005.00771.x. Parasite Immunol. 2005. PMID: 16179031 Review. - Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity.
Hazen SL. Hazen SL. J Biol Chem. 2008 Jun 6;283(23):15527-31. doi: 10.1074/jbc.R700054200. Epub 2008 Feb 19. J Biol Chem. 2008. PMID: 18285328 Free PMC article. Review. No abstract available.
Cited by
- Novel Role of CETP in Macrophages: Reduction of Mitochondrial Oxidants Production and Modulation of Cell Immune-Metabolic Profile.
Dorighello GG, Assis LHP, Rentz T, Morari J, Santana MFM, Passarelli M, Ridgway ND, Vercesi AE, Oliveira HCF. Dorighello GG, et al. Antioxidants (Basel). 2022 Aug 31;11(9):1734. doi: 10.3390/antiox11091734. Antioxidants (Basel). 2022. PMID: 36139808 Free PMC article. - Dendritic Cells and Their Multiple Roles during Malaria Infection.
Amorim KN, Chagas DC, Sulczewski FB, Boscardin SB. Amorim KN, et al. J Immunol Res. 2016;2016:2926436. doi: 10.1155/2016/2926436. Epub 2016 Mar 24. J Immunol Res. 2016. PMID: 27110574 Free PMC article. Review. - Chronicles of Cell Death Foretold: Specificities in the Mechanism of Disposal.
Hughes LD, Bosurgi L, Ghosh S, Rothlin CV. Hughes LD, et al. Front Immunol. 2017 Dec 11;8:1743. doi: 10.3389/fimmu.2017.01743. eCollection 2017. Front Immunol. 2017. PMID: 29312294 Free PMC article. Review. - Monocytes, particularly nonclassical ones, lose their opsonic and nonopsonic phagocytosis capacity during pediatric cerebral malaria.
Vianou B, Royo J, Dechavanne S, Bertin GI, Yessoufou A, Houze S, Faucher JF, Aubouy A. Vianou B, et al. Front Immunol. 2024 May 21;15:1358853. doi: 10.3389/fimmu.2024.1358853. eCollection 2024. Front Immunol. 2024. PMID: 38835780 Free PMC article. - Recruited monocytes modulate malaria-induced lung injury through CD36-mediated clearance of sequestered infected erythrocytes.
Lagassé HA, Anidi IU, Craig JM, Limjunyawong N, Poupore AK, Mitzner W, Scott AL. Lagassé HA, et al. J Leukoc Biol. 2016 May;99(5):659-71. doi: 10.1189/jlb.4HI0315-130RRR. Epub 2015 Oct 29. J Leukoc Biol. 2016. PMID: 26516185 Free PMC article.
References
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. - PubMed
- Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases