The role of DNA shape in protein-DNA recognition - PubMed (original) (raw)
The role of DNA shape in protein-DNA recognition
Remo Rohs et al. Nature. 2009.
Abstract
The recognition of specific DNA sequences by proteins is thought to depend on two types of mechanism: one that involves the formation of hydrogen bonds with specific bases, primarily in the major groove, and one involving sequence-dependent deformations of the DNA helix. By comprehensively analysing the three-dimensional structures of protein-DNA complexes, here we show that the binding of arginine residues to narrow minor grooves is a widely used mode for protein-DNA recognition. This readout mechanism exploits the phenomenon that narrow minor grooves strongly enhance the negative electrostatic potential of the DNA. The nucleosome core particle offers a prominent example of this effect. Minor-groove narrowing is often associated with the presence of A-tracts, AT-rich sequences that exclude the flexible TpA step. These findings indicate that the ability to detect local variations in DNA shape and electrostatic potential is a general mechanism that enables proteins to use information in the minor groove, which otherwise offers few opportunities for the formation of base-specific hydrogen bonds, to achieve DNA-binding specificity.
Figures
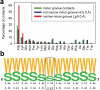
Figure 1. Amino acid frequencies in minor grooves
(a) Histograms for each amino acid illustrate the frequency with which they are observed in any minor groove (green), in minor grooves with a width of ≥5.0 Å (blue), and in narrow minor grooves of <5.0 Å width (red). (b) Frequency of AT (W) and GC (S) base pairs in sequences of 229 sites contacted by arginines in narrow minor grooves. The central base pair (boxed) is contacted by arginine. Frequencies are symmetrized by using both complementary strands.
Figure 2. Distribution of tetranucleotide sequences according to average minor groove width
Tetranucleotides from structures with a minimum length of one helical turn for which minor groove width can be defined are ordered by average minor groove width (red). The widths of all tetranucleotides are shown (black) and the sequence, average width, and occurrence in our dataset are given in Supplementary Table 2. (a) The 59 unique tetranucleotides from free DNA structures. (b) The set of all 136 unique tetranucleotides derived from protein-DNA complexes.
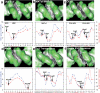
Figure 3. Specific examples of minor groove shape recognition by arginines
DNA shapes of the binding sites of (a) Ubx-Exd 1b8i, (b) MATa1/MATα2 1akh, and (c) Oct-1/PORE 1hf0, (d) the MogR repressor 3fdq, (e) the Tc3 transposase 1u78, and (f) the phage 434 repressor 2or1 are shown in GRASP surface representations, with convex surfaces color-coded in green and concave surfaces in grey/black. Plots of minor groove width (blue) and electrostatic potential in the center of the minor groove (red) are shown below. Arginine contacts (defined by the closest distance between the guanidinium groups and the bases) are indicated. A-tract sequences are highlighted by a solid red line, the TATA box in (e) by a dashed line.
Figure 4. Minor groove shape recognition in the nucleosome
(a) Correlation of minor groove width of the nucleosome core particle (PDB code 1kx5) (blue) and electrostatic potential (red). Arginine contacts (defined by the closest distance between the guanidinium groups and the bases) are indicated. A-tract sequences are highlighted by solid red lines. (b) Schematic representation of the DNA backbone in the nucleosome color-coded by minor groove width (red ≤4.0 Å, pink >4.0 Å and ≤5.0 Å, light blue >5.0 Å and ≤6.0 Å, dark blue >6.0 Å), including all arginines that contact the minor groove. (c) The distribution of A-tracts of length three base pairs or longer in 23,076 yeast nucleosome-bound DNA sequences. (d) Histogram of the occurrence of A-tracts of length three or longer in the same dataset.
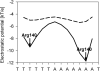
Figure 5. The biophysical origins of the negative potential of narrow minor grooves
Electrostatic potential in the minor groove of the MogR binding site (PDB code 3fdq), calculated in the presence of a dielectric boundary (ε=2 in solute and ε=80 in solvent – solid line) and in the absence of a boundary (ε= 80 in both solute and solvent – dashed line).
Comment in
- Structural biology: DNA binding shapes up.
Tullius T. Tullius T. Nature. 2009 Oct 29;461(7268):1225-6. doi: 10.1038/4611225a. Nature. 2009. PMID: 19865161 No abstract available.
Similar articles
- Electrostatic interactions between arginines and the minor groove in the nucleosome.
West SM, Rohs R, Mann RS, Honig B. West SM, et al. J Biomol Struct Dyn. 2010 Jun;27(6):861-6. doi: 10.1080/07391102.2010.10508587. J Biomol Struct Dyn. 2010. PMID: 20232938 Free PMC article. - Sequence-dependent Kink-and-Slide deformations of nucleosomal DNA facilitated by histone arginines bound in the minor groove.
Wang D, Ulyanov NB, Zhurkin VB. Wang D, et al. J Biomol Struct Dyn. 2010 Jun;27(6):843-59. doi: 10.1080/07391102.2010.10508586. J Biomol Struct Dyn. 2010. PMID: 20232937 Free PMC article. - Origins of specificity in protein-DNA recognition.
Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS. Rohs R, et al. Annu Rev Biochem. 2010;79:233-69. doi: 10.1146/annurev-biochem-060408-091030. Annu Rev Biochem. 2010. PMID: 20334529 Free PMC article. Review. - Recognition of 5'-YpG-3' sequences by coupled stacking/hydrogen bonding interactions with amino acid residues.
Lamoureux JS, Maynes JT, Glover JN. Lamoureux JS, et al. J Mol Biol. 2004 Jan 9;335(2):399-408. doi: 10.1016/j.jmb.2003.10.071. J Mol Biol. 2004. PMID: 14672650 - What drives proteins into the major or minor grooves of DNA?
Privalov PL, Dragan AI, Crane-Robinson C, Breslauer KJ, Remeta DP, Minetti CA. Privalov PL, et al. J Mol Biol. 2007 Jan 5;365(1):1-9. doi: 10.1016/j.jmb.2006.09.059. Epub 2006 Sep 27. J Mol Biol. 2007. PMID: 17055530 Free PMC article. Review.
Cited by
- Decoding dissociation of sequence-specific protein-DNA complexes with non-equilibrium simulations.
van Heesch T, Bolhuis PG, Vreede J. van Heesch T, et al. Nucleic Acids Res. 2023 Dec 11;51(22):12150-12160. doi: 10.1093/nar/gkad1014. Nucleic Acids Res. 2023. PMID: 37953329 Free PMC article. - Structural studies of p53 inactivation by DNA-contact mutations and its rescue by suppressor mutations via alternative protein-DNA interactions.
Eldar A, Rozenberg H, Diskin-Posner Y, Rohs R, Shakked Z. Eldar A, et al. Nucleic Acids Res. 2013 Oct;41(18):8748-59. doi: 10.1093/nar/gkt630. Epub 2013 Jul 17. Nucleic Acids Res. 2013. PMID: 23863845 Free PMC article. - Dissection of Structural Reorganization of Wheat 5B Chromosome Associated With Interspecies Recombination Suppression.
Salina E, Muterko A, Kiseleva A, Liu Z, Korol A. Salina E, et al. Front Plant Sci. 2022 May 4;13:884632. doi: 10.3389/fpls.2022.884632. eCollection 2022. Front Plant Sci. 2022. PMID: 36340334 Free PMC article. - Predicting accurate ab initio DNA electron densities with equivariant neural networks.
Lee AJ, Rackers JA, Bricker WP. Lee AJ, et al. Biophys J. 2022 Oct 18;121(20):3883-3895. doi: 10.1016/j.bpj.2022.08.045. Epub 2022 Sep 3. Biophys J. 2022. PMID: 36057785 Free PMC article. - DNA sequence and chromatin differentiate sequence-specific transcription factor binding in the human malaria parasite Plasmodium falciparum.
Bonnell VA, Zhang Y, Brown AS, Horton J, Josling GA, Chiu TP, Rohs R, Mahony S, Gordân R, Llinás M. Bonnell VA, et al. Nucleic Acids Res. 2024 Sep 23;52(17):10161-10179. doi: 10.1093/nar/gkae585. Nucleic Acids Res. 2024. PMID: 38966997 Free PMC article.
References
- Garvie CW, Wolberger C. Recognition of specific DNA sequences. Mol Cell. 2001;8(5):937–946. - PubMed
- Travers AA. DNA conformation and protein binding. Annu Rev Biochem. 1989;58:427–452. - PubMed
- Shakked Z, et al. Determinants of repressor/operator recognition from the structure of the trp operator binding site. Nature. 1994;368(6470):469–473. - PubMed
- Lu XJ, Shakked Z, Olson WK. A-form conformational motifs in ligand-bound DNA structures. J Mol Biol. 2000;300(4):819–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM054510/GM/NIGMS NIH HHS/United States
- GM54510/GM/NIGMS NIH HHS/United States
- U54 CA121852/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM030518/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases