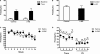The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala - PubMed (original) (raw)
The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala
Brian J Wiltgen et al. Front Behav Neurosci. 2009.
Abstract
Synaptic plasticity in the amygdala is essential for emotional learning. Fear conditioning, for example, depends on changes in excitatory transmission that occur following NMDA receptor activation and AMPA receptor modification in this region. The role of these and other glutamatergic mechanisms have been studied extensively in this circuit while relatively little is known about the contribution of inhibitory transmission. The current experiments addressed this issue by examining the role of the GABA(A) receptor subunit alpha1 in fear learning and plasticity. We first confirmed previous findings that the alpha1 subunit is highly expressed in the lateral nucleus of the amygdala. Consistent with this observation, genetic deletion of this subunit selectively enhanced plasticity in the lateral amygdala and increased auditory fear conditioning. Mice with selective deletion of alpha1 in excitatory cells did not exhibit enhanced learning. Finally, infusion of a alpha1 receptor antagonist into the lateral amygdala selectively impaired auditory fear learning. Together, these results suggest that inhibitory transmission mediated by alpha1-containing GABA(A) receptors plays a critical role in amygdala plasticity and fear learning.
Keywords: emotion; inhibition; long-term potentiation; memory.
Figures
Figure 1
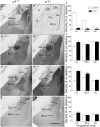
Comparison of labeling for the α1 (A–C), α2 (D–F), α3 (G–I) and α4 (J–L) subunits in three amygdaloid nuclei in coronal sections of control and α1−/− mice. (A) In a control mouse, α1 labeling is high in the lateral amygdaloid nucleus (La) but relatively low in the basolateral nucleus (BLA). Virtually no labeling is evident in the central nucleus (Ce). (B) In a α1−/− mouse, no specific α1 labeling is present in the amygdaloid complex although a few cell bodies are labeled in the globus pallidus. A superimposed schematic drawing identifies the location of the amygdaloid nuclei and nearby structures, including the globus pallidus (GP), caudate putamen (CPu), optic tract (ot) and internal capsule (ic). (D) In a control mouse, moderate α2 subunit labeling is present in the lateral and basolateral nuclei, and strong α2 labeling is evident in the central nucleus. (E) In α1−/− mice, α2 subunit labeling is increased in the lateral nucleus, but no changes are evident in the basolateral and central nuclei. (G) In a control mouse, low levels of α3 subunit labeling are present in the lateral and central nuclei, but moderate α3 labeling is evident in the basolateral nucleus. (H) In a α1−/− mouse, α3 subunit labeling is substantially increased in the lateral nucleus and moderately increased in the basolateral nucleus. (J) In a control mouse, α4 subunit labeling is low in the three amygdaloid nuclei. (K) In a α1−/−mouse, α4 subunit labeling is slightly increased in the lateral nucleus, but remains low in this region, as in the other amygdaloid nuclei. (C, F, I, L) Bar graphs illustrate the virtual absence of α1 labeling in the α1−/− mouse (C), and a significant increase in α2, α3 and α4 labeling (F, I, L) in the lateral nucleus in α1−/− mice. The only other significant change is an increase in α3 labeling in the basolateral nucleus. Error bars represent SEM. **p < 0.01. Scale bar, 500 μm for all panels.
Figure 2
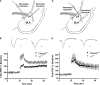
(A) Schematic describing the electrode placement for experiments involving the thalamo-LA pathway. (B) The amount of lateral amygdala activity-dependent plasticity induced by 100 Hz stimulation of the thalamo-LA pathway in slices from floxed+/+ controls (black circles) and α1−/− (white circles) mice. Inset shows sample extracellular traces elicited during baseline (smaller response) and 25–30 min after 100 Hz stimulation in slices from floxed+/+ controls (left side of panel) and α1−/− mice (right side of panel). Calibration bars: 1 ms, 0.1 mV. (C) Schematic describing the electrode placement for experiments involving the LA-BLA pathway. (D) The amount of basolateral amygdala activity-dependent plasticity induced by 100 Hz stimulation of the LA-BLA pathway in slices from floxed+/+ (black circles) and α1−/− (white circles) mice. Inset shows sample extracellular traces elicited during baseline (smaller response) and 25–30 min after 100 Hz stimulation in slices from floxed+/+ (left side of panel) and α1−/− mice (right side of panel). Calibration bars: 1 ms, 0.1 mV.
Figure 3
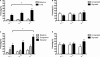
(A) Mice were placed in the training context and received three tone-shock pairings. The next day the animals were placed in a novel environment and received a tone test. All mice showed low levels of baseline freezing and a substantial increase in freezing after the tone was presented. α1−/− mice froze significantly more during the tone than α1+/+ control mice. (B) Mice were placed back into the training environment for a 5-min context test. In addition to the mice that received tone-shock parings (Signaled) we also examined context fear in mice that received three unsignaled shocks during training (Unsignaled). There was no genotype difference in the amount of context freezing. (C) One group of mice were placed in the training context and received five tone-shock pairings (Signaled). Another group of animals received five unsignaled shocks (Unsignaled). The next day the mice received a tone test in a novel environment. All animals showed low levels of baseline freezing and a significant increase in freezing during the tone presentations. The increase in freezing was significantly larger in mice that received signaled training. α1 knockout mice that received signaled training showed substantially more tone fear than α1+/+ control mice. In addition, this fear was specific to the tone paired with shock as α1 knockouts that received unsignaled training froze substantially less. (D) Mice were placed back into the training environment for a 5-min context test. There was no genotype difference in the amount of context freezing.
Figure 4
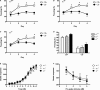
(A) Mice were trained with a white noise and clicker auditory stimulus. For each animal, one of these stimuli was paired with shock (CS+) and the other was not (CS−). Mice received discrimination training across 4 days. Each day, the CS+ and CS− were presented four times in a pseudorandom order (3 min ITI). α1+/+ control mice learned to discriminate across training days and eventually froze more to the CS+ than the CS−. (B) Heterozygous knockout mice learned to discriminate across training days and eventually froze more to the CS+ than the CS−. (C) Homozygous knockout mice learned to discriminate across training days and eventually froze more to the CS+ than the CS−. (D) The day after discrimination training ended mice were given an extinction test. During this test, the CS+ and CS− were each presented four times in the absence of shock. As observed during training, all mice froze significantly more to the CS+ than the CS−. In addition, there was an overall effect of genotype as homozygous knockout mice froze significantly more than heterozygous knockouts and control mice. This increase in freezing did not interact with stimulus type (CS+ or CS−). (E) Acoustic startle was measured over a range of stimuli (0–120 dB) using the MED-ASR-310 testing system. There was a significant effect of volume as startle amplitude increased systematically with increasing dB level. The responses of control mice, heterozygous knockouts and homozygous knockouts were the same across all test stimuli. (F) Mice were next tested on pre-pulse inhibition (PPI). Three pre-pulse intensities were tested: 70, 75, 80 dB. The onset of the pre-pulse preceded the onset of the startle stimulus by 100 ms. The amplitude of the startle response decreased systematically with the intensity of the pre-pulse stimulus. This decrease was the same for all genotypes.
Figure 5
(A) Controls and conditional KO mice were placed in the training context and received three tone-shock pairings. The next daythe animals were placed in a novel environment and received a tone test. Both genotypes mice showed low levels of baseline freezing and a substantial increase in freezing after the tone was presented. There was no genotype difference in the amount of tone fear. (B) Mice were placed back into the training environment for a 5-min context test. There was no genotype difference in the amount of context freezing. (C) Mice received intra-amygdala infusions of saline or 3-PBC and were trained 20 min later. During training the mice received 10 tone-shock. The next day mice were placed back into the training environment for a 10-min context test. There was no difference in the amount of context freezing in mice that were trained with saline and those trained with 3-PBC. (D) The following day the animals were placed in a novel environment and received a tone test. During this test, mice that were trained with 3-PBC froze significantly less than animals trained with saline.
Similar articles
- Plasticity of inhibitory synaptic network interactions in the lateral amygdala upon fear conditioning in mice.
Szinyei C, Narayanan RT, Pape HC. Szinyei C, et al. Eur J Neurosci. 2007 Feb;25(4):1205-11. doi: 10.1111/j.1460-9568.2007.05349.x. Eur J Neurosci. 2007. PMID: 17331216 - Delta Subunit-Containing Gamma-Aminobutyric Acid A Receptor Disinhibits Lateral Amygdala and Facilitates Fear Expression in Mice.
Liu ZP, He QH, Pan HQ, Xu XB, Chen WB, He Y, Zhou J, Zhang WH, Zhang JY, Ying XP, Han RW, Li BM, Gao TM, Pan BX. Liu ZP, et al. Biol Psychiatry. 2017 Jun 15;81(12):990-1002. doi: 10.1016/j.biopsych.2016.06.022. Epub 2016 Jul 6. Biol Psychiatry. 2017. PMID: 27591789 - Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala.
Mahanty NK, Sah P. Mahanty NK, et al. Nature. 1998 Aug 13;394(6694):683-7. doi: 10.1038/29312. Nature. 1998. PMID: 9716132 - Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits.
Maren S. Maren S. Mol Neurobiol. 1996 Aug;13(1):1-22. doi: 10.1007/BF02740749. Mol Neurobiol. 1996. PMID: 8892333 Review. - Molecular mechanisms underlying emotional learning and memory in the lateral amygdala.
Rodrigues SM, Schafe GE, LeDoux JE. Rodrigues SM, et al. Neuron. 2004 Sep 30;44(1):75-91. doi: 10.1016/j.neuron.2004.09.014. Neuron. 2004. PMID: 15450161 Review.
Cited by
- Phenols and GABAA receptors: from structure and molecular mechanisms action to neuropsychiatric sequelae.
Menzikov SA, Zaichenko DM, Moskovtsev AA, Morozov SG, Kubatiev AA. Menzikov SA, et al. Front Pharmacol. 2024 Jan 18;15:1272534. doi: 10.3389/fphar.2024.1272534. eCollection 2024. Front Pharmacol. 2024. PMID: 38303988 Free PMC article. Review. - Prefrontal GABAA(δ)R Promotes Fear Extinction through Enabling the Plastic Regulation of Neuronal Intrinsic Excitability.
Pan HQ, Liu XX, He Y, Zhou J, Liao CZ, You WJ, Jiang SY, Qin X, Chen WB, Fei EK, Zhang WH, Pan BX. Pan HQ, et al. J Neurosci. 2022 Jul 20;42(29):5755-5770. doi: 10.1523/JNEUROSCI.0689-22.2022. Epub 2022 Jun 15. J Neurosci. 2022. PMID: 35705488 Free PMC article. - Corticostriatal control of defense behavior in mice induced by auditory looming cues.
Li Z, Wei JX, Zhang GW, Huang JJ, Zingg B, Wang X, Tao HW, Zhang LI. Li Z, et al. Nat Commun. 2021 Feb 15;12(1):1040. doi: 10.1038/s41467-021-21248-7. Nat Commun. 2021. PMID: 33589613 Free PMC article. - Corticotropin-Releasing Factor Receptor-1 Neurons in the Lateral Amygdala Display Selective Sensitivity to Acute and Chronic Ethanol Exposure.
Agoglia AE, Zhu M, Ying R, Sidhu H, Natividad LA, Wolfe SA, Buczynski MW, Contet C, Parsons LH, Roberto M, Herman MA. Agoglia AE, et al. eNeuro. 2020 Mar 5;7(2):ENEURO.0420-19.2020. doi: 10.1523/ENEURO.0420-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32041742 Free PMC article. - A role for miR-132 in learned safety.
Ronovsky M, Zambon A, Cicvaric A, Boehm V, Hoesel B, Moser BA, Yang J, Schmid JA, Haubensak WE, Monje FJ, Pollak DD. Ronovsky M, et al. Sci Rep. 2019 Jan 24;9(1):528. doi: 10.1038/s41598-018-37054-z. Sci Rep. 2019. PMID: 30679653 Free PMC article.
References
- Blaeser F., Sanders M. J., Truong N., Ko S., Wu L. J., Wozniak D. F., Fanselow M. S., Zhuo M., Chatila T. A. (2006). Long-term memory deficits in Pavlovian fear conditioning in Ca2+/calmodulin kinase kinase alpha-deficient mice. Mol. Cell. Biol. 26, 9105–911510.1128/MCB.01452-06 - DOI - PMC - PubMed
Grants and funding
- R37 AA010422/AA/NIAAA NIH HHS/United States
- P01 NS035985/NS/NINDS NIH HHS/United States
- R01 NS051311/NS/NINDS NIH HHS/United States
- R01 AA010422/AA/NIAAA NIH HHS/United States
- R56 NS051311/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases