Spinocerebellar ataxia type 31 is associated with "inserted" penta-nucleotide repeats containing (TGGAA)n - PubMed (original) (raw)
doi: 10.1016/j.ajhg.2009.09.019. Epub 2009 Oct 29.
Takeshi Amino, Kazuhiro Kobayashi, Shuichi Asakawa, Taro Ishiguro, Taiji Tsunemi, Makoto Takahashi, Tohru Matsuura, Kevin M Flanigan, Sawa Iwasaki, Fumitoshi Ishino, Yuko Saito, Shigeo Murayama, Mari Yoshida, Yoshio Hashizume, Yuji Takahashi, Shoji Tsuji, Nobuyoshi Shimizu, Tatsushi Toda, Kinya Ishikawa, Hidehiro Mizusawa
Affiliations
- PMID: 19878914
- PMCID: PMC2775824
- DOI: 10.1016/j.ajhg.2009.09.019
Spinocerebellar ataxia type 31 is associated with "inserted" penta-nucleotide repeats containing (TGGAA)n
Nozomu Sato et al. Am J Hum Genet. 2009 Nov.
Abstract
Spinocerebellar ataxia type 31 (SCA31) is an adult-onset autosomal-dominant neurodegenerative disorder showing progressive cerebellar ataxia mainly affecting Purkinje cells. The SCA31 critical region was tracked down to a 900 kb interval in chromosome 16q22.1, where the disease shows a strong founder effect. By performing comprehensive Southern blot analysis and BAC- and fosmid-based sequencing, we isolated two genetic changes segregating with SCA31. One was a single-nucleotide change in an intron of the thymidine kinase 2 gene (TK2). However, this did not appear to affect splicing or expression patterns. The other was an insertion, from 2.5-3.8 kb long, consisting of complex penta-nucleotide repeats including a long (TGGAA)n stretch. In controls, shorter (1.5-2.0 kb) insertions lacking (TGGAA)n were found only rarely. The SCA31 repeat insertion's length inversely correlated with patient age of onset, and an expansion was documented in a single family showing anticipation. The repeat insertion was located in introns of TK2 and BEAN (brain expressed, associated with Nedd4) expressed in the brain and formed RNA foci in the nuclei of patients' Purkinje cells. An electrophoretic mobility-shift assay showed that essential splicing factors, serine/arginine-rich splicing factors SFRS1 and SFRS9, bind to (UGGAA)n in vitro. Because (TGGAA)n is a characteristic sequence of paracentromeric heterochromatin, we speculate that the insertion might have originated from heterochromatin. SCA31 is important because it exemplifies human diseases associated with "inserted" microsatellite repeats that can expand through transmission. Our finding suggests that the ectopic microsatellite repeat, when transcribed, might cause a disease involving the essential splicing factors.
Figures
Figure 1
A Comprehensive Physical Map of the 900 kb SCA31 Critical Interval between rs11640843 and −16C > T in the PLEKHG4 Gene This region was entirely covered without gaps by 12 BAC and three fosmid clones derived from a SCA31 homozygous patient. These clones were sequenced. An insertion ranging in length from 2.5–3.8 kb was found at nucleotide number 65,081,803 on human chromosome 16 on NCBI build 36.3 between BEAN and TK2.
Figure 2
Identification of Complex Pentanucleotide Repeat Insertions in SCA31 Patients (A) Southern blot analysis showing the SCA31 insertion. The left-hand panel shows EcoRI-digested genomic fragments detected with a cosmid probe for the region between nucleotides 65,083,571 and 65,124,051. A rare 1.5 kb insertion and an unusual 0.7 kb expanded (TAAAA)n (both shown with solid black arrows) were observed in one control (control 1). SCA31 insertions in two patients are shown with a red arrow. “Hom.” and “Het.” designate the homozygous patient and heterozygous patient, respectively. The dotted arrow indicates normal chromosomes without insertions. The thick 5.8 kb bands common in the three subjects show fragments outside the insertion site. The right-hand panel shows aberrant EcoRI-digested 9–10 kb genomic fragments (a red arrow) that completely segregated with SCA31 patients (P). All heterozygous patients (P) and controls (C) have “normal” 6 kb fragments (dotted arrows). Radiolabeled PCR products obtained by amplifying the 3009 bp genomic segment between nucleotides 65,079,127 and 65,082,135 on NCBI build 36.3 were used as probes. (B) Sequences around the SCA31 insertion (chromosome16: nucleotides 65,081,260–65,082,786 on NCBI build 36.3). Flanking primers for PCR amplification (underlined in red) of insertion and flanking HaeIII recognition sites (in shaded boxes) are shown. The AluSx sequence is shown with a green underline. Without an insertion, PCR amplification with flanking primers and a subsequent HaeIII digestion will produce a DNA fragment 193 bp in length. (C) The components of the SCA31 insertion in the homozygous patient. The SCA31 insertion consists of a preceding 4 bp TCAC and three different penta-nucleotides, (TGGAA)n, (TAGAA)n, and (TAAAA)n. (TGGAA)n is the patient-specific repeat (shown in red), and both (TGGAA)n and (TAAAATAGAA)n are pure stretches too long to be read through. The bridging sequence between (TGGAA)n and (TAGAA)46 is underlined in red. (D) The sequence of the insertion in control 1. Rare insertions were observed in controls at the same position as the SCA31 insertion, but with shorter length and different components. The insertion in control 1 consisted of a preceding 4 bp TCAC and two pentanucleotide components, (TAGAA)n and (TAAAA)n. The (TGGAA)n was not detected.
Figure 3
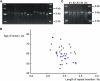
The Length of Insertion Inversely Correlates with Age of Onset in SCA31 (A) PCR amplification and agarose gel electrophoresis showing that the length of the insertion differs among SCA31 families. (B) A scatter plot showing an inverse correlation between the length of the SCA31 insertion and the age of onset. The length of the repeat insertion was inversely correlated with age of onset (Pearson's product-moment correlation coefficient r = −0.41, p = 0.010, n = 39.). (C) A slight expansion of the SCA31 insertion observed in one SCA31 family. Individual #4 has a slightly longer insertion than the others (#1–#3) in the same SCA31 family. This individual #4 is in the youngest generation among them.
Figure 4
Various Transcripts Spanning the SCA31 Repeat Insertion Site The locations of BEAN, TK2, FLJ27243, and the SCA31 insertion (red arrowhead) are shown on the physical map of the chromosomal region between nucleotides 65,000,000 and 65,150,000 on NCBI build 36.3. Exons registered in the NCBI database (shown in a shaded area) are shown with black boxes, and 5′- and 3′-UTRs are shown with white boxes attached to them. Although the SCA31 insertion is located in the intergene region between BEAN and TK2 on the NCBI database, various newly identified transcripts of these genes (shown with white boxes with their exon numbers) were detected by RT-PCR, and some of them encompassed the SCA31 insertion. The insertion appeared to be located in introns of BEAN and TK2, two genes transcribed in opposite directions. DA392036 annotated in the NCBI database seemed to be a part of TK2-EXT. Transcripts 1–3 correspond to the transcripts detected by RT-PCR in Figures 5A and 5B (Table S1). The primer pairs for RT-PCR are shown with small blue boxes (A–J; Figure 5D and Tables S1 and S2).
Figure 5
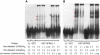
Gene Expression of BEAN-EXT, TK2-EXT, and FLJ27243 in Humans (A) RT-PCR analysis for BEAN-EXT mRNA (transcripts 1 and 2 in Figure 5) showing its brain-specific expression. (B) RT-PCR analysis for TK2-EXT mRNA (transcript 3 in Figure 5) showing higher expression in various systemic organs than in the brain. (C) RT-PCR of FLJ27243 mRNA in the human cerebellum. Strand-specific RT-PCR shows expression of FLJ27243, represented by “RT with AS primer,” in the brain. The “S primer” represents transcription in the orientation of BEAN, and “Random Hexamer” indicates transcripts in both directions. The specificity of this strand-specific RT-PCR is confirmed by negative amplification when reverse transcriptase is omitted [(-)]. (D) Quantitative RT-PCR on BEAN, TK2, FLJ27243, and CKLF mRNAs in controls' (n = 4) and patients' (n = 2) cerebella. The locations of RT-PCR primer and probe sets (A–J) are indicated in Figure 5 (C: controls; P: patients; the scale bar represents 1 SD) (see Table S2 for probe sequences). No consistent difference was found in the expression levels of BEAN (including BEAN-EXT; probe sets: A–G), TK2 (probe sets H and I), FLJ27243 (probe set J), or CKLF (probe sets K–L) mRNAs compared in the control versus SCA31 patient groups.
Figure 6
Presence of RNA Foci in SCA31 Purkinje Cells RNA foci (red dots) seen in a nucleus (stained with DAPI; blue) of an SCA31 Purkinje cell with an LNA-(TTTTATTCTA)2.5 probe targeting the transcripts containing the (UAAAAUAGAA)n repeat (autofluorescence; orange). In controls, foci were completely negative. Anti-sense transcripts, searched with an LNA-(TAGAATAAAA)2.5 probe, did not appear as RNA foci. Scale bars represent 10 μm.
Figure 7
The Pentanucleotide (TGGAA)n Binds to Splicing Factors SFRS1 and SFRS9 In Vitro EMSA showing specific binding of SFRS1 isoform 1 (SFRS1-1) (A) and SFRS9 (B) to RNA oligonucleotide (UGGAA)8. Shifted bands (arrowheads) were observed in mixtures of digoxigenin(DIG)-(UGGAA)8 and either GST-SFRS1-1 or GST-SFRS-9. The shifted bands disappeared with the addition of nonlabeled (UGGAA)8, whereas the addition of excess amounts of nonlabeled (UAGAA)8 or (UAAAAUAGAA)4 did not interfere with the band shift. No shift was seen when DIG-(UGGAA)8 was mixed with GST alone.
Figure 8
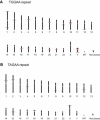
(TGGAA)n Is Particularly Abundant in Centromeric Regions (A) (TGGAA)40 sequences are abundant in the centromeres of chromosomes 2, 4, 7, 10, 16, 17, 20 and Y, whereas they are sparse in normal euchromosomes. (B) (TAGAA)40 sequences are widely observed in euchromatin as well as in telomeres.
Similar articles
- [Spinocerebellar ataxia type 31].
Ishikawa K, Sato N, Niimi Y, Amino T, Mizusawa H. Ishikawa K, et al. Rinsho Shinkeigaku. 2010 Nov;50(11):985-7. doi: 10.5692/clinicalneurol.50.985. Rinsho Shinkeigaku. 2010. PMID: 21921537 Review. Japanese. - Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)n in the Purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis.
Niimi Y, Takahashi M, Sugawara E, Umeda S, Obayashi M, Sato N, Ishiguro T, Higashi M, Eishi Y, Mizusawa H, Ishikawa K. Niimi Y, et al. Neuropathology. 2013 Dec;33(6):600-11. doi: 10.1111/neup.12032. Epub 2013 Apr 22. Neuropathology. 2013. PMID: 23607545 - Molecular Mechanisms and Future Therapeutics for Spinocerebellar Ataxia Type 31 (SCA31).
Ishikawa K, Nagai Y. Ishikawa K, et al. Neurotherapeutics. 2019 Oct;16(4):1106-1114. doi: 10.1007/s13311-019-00804-6. Neurotherapeutics. 2019. PMID: 31755042 Free PMC article. Review. - Analysis of an insertion mutation in a cohort of 94 patients with spinocerebellar ataxia type 31 from Nagano, Japan.
Sakai H, Yoshida K, Shimizu Y, Morita H, Ikeda S, Matsumoto N. Sakai H, et al. Neurogenetics. 2010 Oct;11(4):409-15. doi: 10.1007/s10048-010-0245-6. Epub 2010 Apr 28. Neurogenetics. 2010. PMID: 20424877 Free PMC article. - Spinocerebellar ataxia type 31 (SCA31).
Ishikawa K. Ishikawa K. J Hum Genet. 2023 Mar;68(3):153-156. doi: 10.1038/s10038-022-01091-4. Epub 2022 Nov 1. J Hum Genet. 2023. PMID: 36319738 Free PMC article. Review.
Cited by
- Dynamic changes of nuclear RNA foci in proliferating DM1 cells.
Xia G, Ashizawa T. Xia G, et al. Histochem Cell Biol. 2015 Jun;143(6):557-64. doi: 10.1007/s00418-015-1315-5. Epub 2015 Feb 26. Histochem Cell Biol. 2015. PMID: 25715678 Free PMC article. - SMRT Sequencing of Long Tandem Nucleotide Repeats in SCA10 Reveals Unique Insight of Repeat Expansion Structure.
McFarland KN, Liu J, Landrian I, Godiska R, Shanker S, Yu F, Farmerie WG, Ashizawa T. McFarland KN, et al. PLoS One. 2015 Aug 21;10(8):e0135906. doi: 10.1371/journal.pone.0135906. eCollection 2015. PLoS One. 2015. PMID: 26295943 Free PMC article. - Origin and evolution of pentanucleotide repeat expansions at the familial cortical myoclonic tremor with epilepsy type1 - SAMD12 locus.
Chen X, Zhang F, Shi Y, Wang H, Chen M, Yang D, Wang L, Liu P, Xie F, Chen J, Fu A, Hu B, Wang B, Ouyang Z, Wu S, Lin Z, Cen Z, Luo W. Chen X, et al. Eur J Hum Genet. 2024 Mar 12. doi: 10.1038/s41431-024-01586-y. Online ahead of print. Eur J Hum Genet. 2024. PMID: 38467733 - Structure of native four-repeat satellite III sequence with non-canonical base interactions.
Chen E, Trajkovski M, Lee HK, Nyovanie S, Martin KN, Dean WL, Tahiliani M, Plavec J, Yatsunyk LA. Chen E, et al. Nucleic Acids Res. 2024 Apr 12;52(6):3390-3405. doi: 10.1093/nar/gkae113. Nucleic Acids Res. 2024. PMID: 38381082 Free PMC article. - Genome-wide detection of tandem DNA repeats that are expanded in autism.
Trost B, Engchuan W, Nguyen CM, Thiruvahindrapuram B, Dolzhenko E, Backstrom I, Mirceta M, Mojarad BA, Yin Y, Dov A, Chandrakumar I, Prasolava T, Shum N, Hamdan O, Pellecchia G, Howe JL, Whitney J, Klee EW, Baheti S, Amaral DG, Anagnostou E, Elsabbagh M, Fernandez BA, Hoang N, Lewis MES, Liu X, Sjaarda C, Smith IM, Szatmari P, Zwaigenbaum L, Glazer D, Hartley D, Stewart AK, Eberle MA, Sato N, Pearson CE, Scherer SW, Yuen RKC. Trost B, et al. Nature. 2020 Oct;586(7827):80-86. doi: 10.1038/s41586-020-2579-z. Epub 2020 Jul 27. Nature. 2020. PMID: 32717741 Free PMC article.
References
- Brice A., Pulst S.-M. Butterworth Heinemann, Elsevier, Inc.; Philadelphia: 2007. Spinocerebellar degenerations: The ataxias and spastic paraplegias.
- Zoghbi H.Y., Orr H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 2000;23:217–247. - PubMed
- Ranum L.P.W., Cooper T.A. RNA-mediated neuromuscular disorders. Annu. Rev. Neurosci. 2006;29:259–277. - PubMed
- Bandmann O., Singleton A.B. Yet another spinocerebellar ataxia: The saga continues. Neurology. 2008;71:542–543. - PubMed
- Nagaoka U., Takashima M., Ishikawa K., Yoshizawa K., Yoshizawa T., Ishikawa M., Yamawaki T., Shoji S., Mizusawa H. A gene on SCA4 locus causes dominantly inherited pure cerebellar ataxia. Neurology. 2000;54:1971–1975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous