Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice - PubMed (original) (raw)
Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice
Edwin F de Zoeten et al. Gastroenterology. 2010 Feb.
Abstract
Background & aims: Foxp3+ T regulatory cells (Tregs) help prevent autoimmunity, and increases in their numbers of functions could decrease the development of inflammatory bowel disease. Like other cells, Foxp3+ Tregs express histone/protein deacetylases (HDACs), which regulate chromatin remodeling and gene expression. We investigated whether disruption of a specific class IIa HDAC, HDAC9, activity in Tregs affects the pathogenesis of colitis in mice.
Methods: We tested the effects of various HDAC inhibitors (HDACi) in models of colitis using wild-type mice. We also transferred Tregs and non-Treg cells from HDAC9-/- or wild-type mice to immunodeficient mice. HDAC9 contributions to the functions of Tregs were determined during development and progression of colitis.
Results: Pan-HDACi, but not class I-specific HDACi, increased the functions of Foxp3+ Tregs, prevented colitis, and reduced established colitis in mice, indicating the role of class II HDACs in controlling Treg function. The abilities of pan-HDACi to prevent/reduce colitis were associated with increased numbers of Foxp3+ Tregs and their suppressive functions. Colitis was associated with increased local expression of HDAC9; HDAC9-/- mice resistant to development of colitis. HDAC9-/- Tregs expressed increased levels of the heat shock protein (HSP) 70, compared with controls. Immunoprecipitation experiments indicated an interaction between HSP70 and Foxp3. Inhibition of HSP70 reduced the suppressive functions of HDAC9-/- Tregs; Tregs that overexpressed HSP70 had increased suppressive functions.
Conclusions: Strategies to decrease HDAC9 expression or function in Tregs or to increase expression of HSP70 might be used to treat colitis and other autoimmune disorders.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures
Figure 1
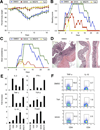
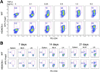
Contrasting effects of pan-HDACi and class I-specific HDACi therapy on colitis. Mice (n = 10/group) receiving DSS plus TsA, SAHA, MS275, or DMSO carrier alone were evaluated daily for (A) weight loss (mean ± SD), (B) bleeding, and (C) stool consistency. Mice receiving TsA or SAHA had less weight loss, stool blood, or diarrhea (P < .01) than mice treated with MS275 or DMSO. (D) H&E-stained colonic sections (original magnification, ×125) from mice receiving DSS and treatment for 10 days with TsA dissolved in DMSO or DMSO alone (representative of n = 6/group). (E) qPCR analysis of colons from normal mice or those receiving DSS or DSS plus TsA, SAHA, or MS75 at day 10 after initiation of DSS therapy; data (mean ± SD, n = 6/group) are expressed as fold increase compared with levels in normal WT colons and were normalized to 18S; Foxp3, P < .01 for TsA or SAHA vs DMSO or MS275; IL-10, P < .05 for TsA and P < .01 for SAHA vs control or MS275; IL-2,P < .005 for TsA or SAHA vs control or MS275; IFN-γ, P < .02 for TsA or SAHA vs control or MS275; TNF-α, P < .01 for TsA or SAHA vs control of MS275; and TGF-β, P < .05 for SAHA vs MS275; differences in TGF-β expression between DSS or TsA were not significant (P < .05). (F) Intracellular staining of lamina propria mononuclear cells on day 10 shows a decreased proportion of cells expressing TNF-α (P < .05) and an increased proportion of cells expressing IL-10 (P < .01) in mice receiving TSA or SAHA (representative of n = 6 mice/group).
Figure 2
Contrasting effects of pan-HDACi and class I-specific HDACi therapy on Treg function in vivo. After 7 days of in vivo therapy in normal C57BL/6 mice using MS275 or SAHA, CD4+CD25+ cells were isolated using magnetic beads and added to cultures of CFSE-labeled CD4+CD25− T cells that were activated with CD3 mAb and irradiated antigen-presenting cells. The percentage of proliferating T cells is shown in each plot, and data are representative of 3 experiments.
Figure 3
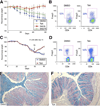
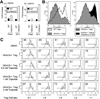
Pan-HDACi therapy and prevention or treatment of T cell-induced colitis. Panels A and B show prevention data, and panels C and E involve treatment once colitis had developed. (A) Rag1−/− mice (mean ± SD, n = 8/group) adoptively transferred with 1 × 106 C57BL/6 CD4+CD25− cells and treated with TsA (yellow) or SAHA (red) did not develop colitis, in contrast to mice treated with MS275 (green, P < .03) or DMSO alone (_blue, P_ < .01). (_B_) Flow cytometry of mesenteric LN cells harvested at 14 days showed increased Foxp3+ Tregs in TsA-treated mice; data representative of n = 8 mice/group. (_C_) Rag1−/− mice (mean ± SD, n = 8/group) adoptively transferred with 1 × 106 C57BL/6 CD4+CD25− cells developed clinical evidence of colitis and >20% weight loss by ~55 days posttransfer. In contrast to use of DMSO alone (blue), mice treated with TsA (red) showed clinical improvement (P < .005). (D) Flow cytometry of mesenteric LN cells harvested at 14 days after onset of therapy showed increase in Foxp3+ Tregs in TsA-treated mice; data representative of n = 8/group. (E and F) Representative histology (n = 8/group, Alcian blue; original magnification, ×125) after 3 weeks of therapy with DMSO (E) vs TsA (F), showing differential effects on leukocyte infiltration, goblet cell loss, and submucosa thickening.
Figure 4
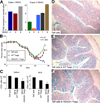
HDAC9 and colitis. (A) qPCR analysis of HDAC expression in colons of DSS-treated mice; data (mean ± SD, n = 6/group) expressed as fold increase over levels in normal colons (after normalization to 18S). (B) Rag−/− mice were injected with 1 × 106 WT CD4+CD25− naïve T cells, and weights (mean ± SD, n = 8/group) were measured at least twice weekly. Once colitis had developed, mice were injected with 5 × 105 CD4+CD25+ cells from naïve C57BL6 (red) or HDAC9−/− mice (green) or with control naïve CD4+CD25− cells (blue); **P < .005 for WT Tregs vs Teff cells and *P < .01 for HDAC9−/− vs WT Tregs. (C) Flow cytometric quantitation of splenocyte and mesenteric lymph node (MLN) cell numbers (mean ± SD, n = 4 mice/group) at 21 days after injection of CD4+CD25+ cells from HDAC9−/− vs WT Tregs (*P < .01). (D–F) Representative histology (n = 8/group) at 21 days posttransfer of indicated cell populations (Alcian blue; original magnification, ×100).
Figure 5
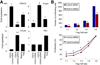
HDAC9 knockdown and Foxp3+ Treg suppression. (A) qPCR analysis (mean ± SD, n = 4/group) of effects of HDAC9 knockdown on gene expression in resting and activated in naïve CD4+CD25+ cells; data normalized relative to 18S (*P < .05 and **P < .01 vs use of control siRNA). (B) Effects of HDAC9 knockdown on Treg function in vitro; data shown as number of proliferating cells (upper panel) and as percentage proliferating cells at each ratio (lower panel); data representative of 4 experiments (*P < .05 vs corresponding control siRNA value).
Figure 6
Conversion of HDAC9−/− vs WT CD4+CD25− cells. (A) WT and HDAC9−/− CD4+CD25− cells were cultured for 3 days with plate-bound CD3 and CD28 mAbs, plus IL-2 (10 U/mL) and increasing concentrations of TGF-α; the percentages of CD4+Foxp3+ cells are shown and are representative of 4 experiments. (B) WT and HDAC9−/− CD4+CD45RBhi CD4+CD25− cells (1 × 106 cells) were injected IP into Rag1−/− mice; spleen, mesenteric lymph node (MLN), and peripheral lymph node (LN) samplers harvested at the weeks indicated were analyzed by flow cytometry; data representative of 4 experiments.
Figure 7
Pharmacologic effects of HSP70 inhibition in Tregs. (A) qPCR analysis of HSP40 and HSP70 expression by resting or activated Tregs (using CD3/CD28 mAbs) from HDAC9−/− and WT mice; data shown as fold increase, normalized relative to 18S and representative of 3 experiments. (B) Flow cytometric analysis of WT or HDAC9−/− Treg cell apoptosis as shown by Annexin-V staining. Left panel shows Annexin-V staining of the cell populations after culture for 24 hours with CD3 mAb and IL-2. Right panel shows corresponding analysis of HDAC9−/− cells cultured with or without addition of Triptolide (data representative of 3 experiments). (C) Inhibitory effects of increasing concentrations of Triptolide on the functions of HDAC9−/− Tregs in vitro using a standard Treg suppression assay involving proliferation of CFSE-labeled T cells; percentage of CFSE+ cells in each well is indicated (data representative of 3 experiments).
Figure 8
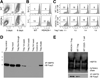
HSP70 and Foxp3 expression in Tregs. (A) Comparison of Treg conversion at 3 and 6 days of culture of HDAC9−/− and WT T cells in presence of CD3 mAb (1 µg/mL), TGF-β (3 ng/mL), and IL-2 (5 U/mL). (B) Comparison of proliferation of WT and HDAC9−/− CFSE-labeled Tregs cultured for 3 days with CD3 mAb and irradiated APC, with or without IL-2 (5 U/mL) as indicated. (C) Assessment of Treg proliferation under Treg assay-like conditions. CFSE-labeled CD4+CD25+ T cells and unlabeled CD4+CD25− T cells (Teff) cells were stimulated for 72 hours with CD3 mAb (0.5 µg/mL) plus 4 × 105 irradiated APC; percentage of CFSE-labeled CD4+CD25+ T cells at each ratio of Teff to Treg is indicated. (D) Immunoprecipitation of HSP70 from WT Tregs leads to coprecipitation of Foxp3 (47 kilodaltons, lowermost molecular weight marker). (E) Increased coprecipitation of HSP70 and Foxp3 using HDAC9−/− vs WT Tregs and lack of coprecipitation of HSP70 and Foxp3 when using CD4+CD25− T cells. In panels A–E, data are representative of 3 experiments.
Similar articles
- Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms.
Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Beier UH, et al. Sci Signal. 2012 Jun 19;5(229):ra45. doi: 10.1126/scisignal.2002873. Sci Signal. 2012. PMID: 22715468 Free PMC article. - Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW. de Zoeten EF, et al. Mol Cell Biol. 2011 May;31(10):2066-78. doi: 10.1128/MCB.05155-11. Epub 2011 Mar 28. Mol Cell Biol. 2011. PMID: 21444725 Free PMC article. - Gut Inflammation in Mice Triggers Proliferation and Function of Mucosal Foxp3+ Regulatory T Cells but Impairs Their Conversion from CD4+ T Cells.
Boschetti G, Kanjarawi R, Bardel E, Collardeau-Frachon S, Duclaux-Loras R, Moro-Sibilot L, Almeras T, Flourié B, Nancey S, Kaiserlian D. Boschetti G, et al. J Crohns Colitis. 2017 Jan;11(1):105-117. doi: 10.1093/ecco-jcc/jjw125. Epub 2016 Jun 30. J Crohns Colitis. 2017. PMID: 27364948 - Histone deacetylase inhibitors and transplantation.
Tao R, de Zoeten EF, Ozkaynak E, Wang L, Li B, Greene MI, Wells AD, Hancock WW. Tao R, et al. Curr Opin Immunol. 2007 Oct;19(5):589-95. doi: 10.1016/j.coi.2007.07.015. Epub 2007 Aug 24. Curr Opin Immunol. 2007. PMID: 17719760 Free PMC article. Review. - Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells.
Beier UH, Akimova T, Liu Y, Wang L, Hancock WW. Beier UH, et al. Curr Opin Immunol. 2011 Oct;23(5):670-8. doi: 10.1016/j.coi.2011.07.002. Epub 2011 Jul 26. Curr Opin Immunol. 2011. PMID: 21798734 Free PMC article. Review.
Cited by
- Histone deacetylase 3 regulates the inflammatory gene expression programme of rheumatoid arthritis fibroblast-like synoviocytes.
Angiolilli C, Kabala PA, Grabiec AM, Van Baarsen IM, Ferguson BS, García S, Malvar Fernandez B, McKinsey TA, Tak PP, Fossati G, Mascagni P, Baeten DL, Reedquist KA. Angiolilli C, et al. Ann Rheum Dis. 2017 Jan;76(1):277-285. doi: 10.1136/annrheumdis-2015-209064. Epub 2016 Jul 25. Ann Rheum Dis. 2017. PMID: 27457515 Free PMC article. - Histone acetyltransferases and histone deacetylases in B- and T-cell development, physiology and malignancy.
Haery L, Thompson RC, Gilmore TD. Haery L, et al. Genes Cancer. 2015 May;6(5-6):184-213. doi: 10.18632/genesandcancer.65. Genes Cancer. 2015. PMID: 26124919 Free PMC article. Review. - IL-4 inhibits regulatory T cells differentiation by HDAC9-mediated epigenetic regulation.
Cui J, Xu H, Yu J, Li Y, Chen Z, Zou Y, Zhang X, Du Y, Xia J, Wu J. Cui J, et al. Cell Death Dis. 2021 May 18;12(6):501. doi: 10.1038/s41419-021-03769-7. Cell Death Dis. 2021. PMID: 34006836 Free PMC article. - The tumor suppressor kinase LKB1 activates the downstream kinases SIK2 and SIK3 to stimulate nuclear export of class IIa histone deacetylases.
Walkinshaw DR, Weist R, Kim GW, You L, Xiao L, Nie J, Li CS, Zhao S, Xu M, Yang XJ. Walkinshaw DR, et al. J Biol Chem. 2013 Mar 29;288(13):9345-62. doi: 10.1074/jbc.M113.456996. Epub 2013 Feb 7. J Biol Chem. 2013. PMID: 23393134 Free PMC article. - HDAC1 controls CD8+ T cell homeostasis and antiviral response.
Tschismarov R, Firner S, Gil-Cruz C, Göschl L, Boucheron N, Steiner G, Matthias P, Seiser C, Ludewig B, Ellmeier W. Tschismarov R, et al. PLoS One. 2014 Oct 21;9(10):e110576. doi: 10.1371/journal.pone.0110576. eCollection 2014. PLoS One. 2014. PMID: 25333902 Free PMC article.
References
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. - PubMed
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. - PubMed
- Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. - PubMed
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK080189/DK/NIDDK NIH HHS/United States
- P01AI073489/AI/NIAID NIH HHS/United States
- P01 AI073489/AI/NIAID NIH HHS/United States
- K08DK080189/DK/NIDDK NIH HHS/United States
- R01AI073938/AI/NIAID NIH HHS/United States
- R01 AI073938/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources