Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer - PubMed (original) (raw)
Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer
Shahana Majid et al. Cancer. 2010.
Abstract
Background: : B-cell translocation gene 3 (BTG3/ANA/APRO4) is a candidate tumor suppressor gene in some malignancies. We report here that B-cell translocation gene 3 (BTG3) is transcriptionally down-regulated in prostate cancer and the mechanism of inactivation is through promoter hypermethylation.
Methods: : Prostate cancer and normal cell lines were treated with different doses of genistein and 5-aza-2'-deoxycytidine (5Aza-C). BTG3 messenger ribonucleic acid (mRNA) expression was determined by quantitative real-time polymerase chain reaction in tissues and cell lines. Bisulfate-modified polymerase chain reaction, cloning and sequencing were used to examine promoter methylation in tumor samples and cell lines. Enzyme activity/inhibition assays were done to check the effect of genistein and 5Aza-C on DNA methyltransferases. ChIP assay was performed to analyze chromatin modifications caused by genistein treatment.
Results: : BTG3 mRNA expression was down-regulated in cancer tissues and cells. Genistein and 5Aza-C induced BTG3 mRNA expression in all PC cell lines. Complete methylation of BTG3 promoter in tumor samples and cancer cell lines was observed. Genistein and 5Aza-C treatment significantly decreased promoter methylation, reactivating BTG3 expression. Genistein and 5Aza-C increased levels of acetylated histones 3, 4, histone 3 dimethylated at lysine 4, histone 3 trimethylated at lysine 4, and RNA polymerase II, decreased DNA methyl transferase and methyl-binding domain protein 2 activity, and increased histone acetyl transferase (HAT) activity.
Conclusions: : This is the first report to show that BTG3 is silenced in prostate cancer and can be reactivated by genistein-induced promoter demethylation and active histone modification. Genistein showed similar effects to that of 5Aza-C, which is currently undergoing phase 2 clinical trials as a treatment for prostate cancer. Because genistein is a natural, nontoxic, and dietary isoflavone, these results indicate that genistein is a novel, advantageous therapeutic agent for treating prostate cancer.
Copyright 2010 American Cancer Society.
Figures
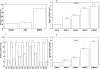
Figure 1
A, Expression profile of BTG3 in prostate cancer and normal prostate epithelial cells. C, Expression profile of BTG3 in prostate cancer (T) and normal prostate (N) clinical samples. B, D, Relative expression profile of BTG3 gene following treatment with 0, 10, 25 and 50uM genistein (G) and 5uM 5-Aza-2’-deoxycytidine (Aza). Relative quantification was performed by quantitative real-time PCR. Data are in triplicate from three independent experiments and were normalized to GAPDH and calibrated to levels in untreated samples. All data are expressed as mean ±SE (bars). * Statistically significant at p<0.05.
Figure 2
A, Graphic depiction of the CpG islands in the promoter of BTG3 gene. B, Position and sequence of primers used for BSP and ChIP.
Figure 3
A, DNA sequencing results showing promoter methylation status in untreated LNCaP, PC3 and RWPE-1 cells. B, Representative figures showing methylation status in BPH, normal and tumor prostate tissues. * indicates individual CpG sites. For cell lines, a single primer set (BSP_F3 –R3) was used for BS-PCR whereas a nested PCR was performed for tissue samples using two primer sets BSP_F1 – R1 and BSP_F2 – R2.
Figure 4
A, Representative sequencing results showing the effect of 5Aza-C and genistein on promoter methylation. B, Summarized results showing demethylation of CpG sites by 5Aza-C and genistein treatments in LNCaP and PC3 cell lines. C, Summarized results showing promoter methylation status in 10 BPH samples and in 10 pairs of normal and tumor samples. Each pair was obtained from the same patient and micro-dissected by a certified pathologist into normal and tumor. D, Summarized sequencing results from 10 clones of each selected sample. Each row represents a single clone. ○, * indicate individual CpG sites in the CpG island. For cloning samples a nested PCR was performed using two primer sets BSP_F1 – R1 and BSP_F3 – R3.
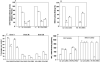
Figure 5
A, Percent Methyltransferase Activity. B, MBD2 binding Activity expressed as binding percent. C, DNMT proteins expressed as percent decrease over control calculated by formula [(Treated/untreated control) × 100 – 100]. D, HAT and HDAC activity (ng/h/ml). +C positive control provided with the assay kit. LNCaP (L), PC3 (P), Genistein (G), 5Aza-C (5A), untreated control (0), 50uM Genistein (50G), 5uM 5Aza-C (5A).
Figure 6
A. Histone modifications. ChIP assay was performed on cells after treatment with 50uM/L genistein and 5uM/L 5Aza-C. Untreated control (0), 5Aza-C (5A), 50uM Genistein (50G). B. Data calculated from the corresponding DNA fragments amplified by PCR at annealing temperature of 60°C for a total of 28 cycles; bars, error ±SD. Enrichment was calculated as the ratio between the net intensity of each bound sample normalized to its input sample and the vehicle control sample normalized to vehicle control input samples (Bound sample/Bound sample Input)/(Vehicle control sample/Vehicle control Input).
Similar articles
- BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer.
Majid S, Dar AA, Ahmad AE, Hirata H, Kawakami K, Shahryari V, Saini S, Tanaka Y, Dahiya AV, Khatri G, Dahiya R. Majid S, et al. Carcinogenesis. 2009 Apr;30(4):662-70. doi: 10.1093/carcin/bgp042. Epub 2009 Feb 12. Carcinogenesis. 2009. PMID: 19221000 Free PMC article. - PCDH17 gene promoter demethylation and cell cycle arrest by genistein in gastric cancer.
Yang Y, Liu J, Li X, Li JC. Yang Y, et al. Histol Histopathol. 2012 Feb;27(2):217-24. doi: 10.14670/HH-27.217. Histol Histopathol. 2012. PMID: 22207556 - Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells.
Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Majid S, Igawa M, Dahiya R. Kikuno N, et al. Int J Cancer. 2008 Aug 1;123(3):552-60. doi: 10.1002/ijc.23590. Int J Cancer. 2008. PMID: 18431742 Retracted. - Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification.
Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, Hirata H, Li LC, Zhao H, Okino ST, Place RF, Pookot D, Dahiya R. Majid S, et al. Cancer Res. 2008 Apr 15;68(8):2736-44. doi: 10.1158/0008-5472.CAN-07-2290. Cancer Res. 2008. PMID: 18413741 - The epigenome as a therapeutic target in prostate cancer.
Perry AS, Watson RW, Lawler M, Hollywood D. Perry AS, et al. Nat Rev Urol. 2010 Dec;7(12):668-80. doi: 10.1038/nrurol.2010.185. Epub 2010 Nov 9. Nat Rev Urol. 2010. PMID: 21060342 Review.
Cited by
- Impact of Epigenetic Dietary Components on Cancer through Histone Modifications.
Gao Y, Tollefsbol TO. Gao Y, et al. Curr Med Chem. 2015;22(17):2051-64. doi: 10.2174/0929867322666150420102641. Curr Med Chem. 2015. PMID: 25891109 Free PMC article. Review. - DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions.
Jasek K, Kubatka P, Samec M, Liskova A, Smejkal K, Vybohova D, Bugos O, Biskupska-Bodova K, Bielik T, Zubor P, Danko J, Adamkov M, Kwon TK, Büsselberg D. Jasek K, et al. Biomolecules. 2019 Jul 18;9(7):289. doi: 10.3390/biom9070289. Biomolecules. 2019. PMID: 31323834 Free PMC article. Review. - Natural Bioactive Compounds Targeting Histone Deacetylases in Human Cancers: Recent Updates.
Bouyahya A, El Hachlafi N, Aanniz T, Bourais I, Mechchate H, Benali T, Shariati MA, Burkov P, Lorenzo JM, Wilairatana P, Mubarak MS, El Omari N. Bouyahya A, et al. Molecules. 2022 Apr 15;27(8):2568. doi: 10.3390/molecules27082568. Molecules. 2022. PMID: 35458763 Free PMC article. Review. - Cancer chemoprevention by dietary polyphenols: promising role for epigenetics.
Link A, Balaguer F, Goel A. Link A, et al. Biochem Pharmacol. 2010 Dec 15;80(12):1771-92. doi: 10.1016/j.bcp.2010.06.036. Epub 2010 Jun 26. Biochem Pharmacol. 2010. PMID: 20599773 Free PMC article. Review. - Inhibition of Cancer Development by Natural Plant Polyphenols: Molecular Mechanisms.
Lyubitelev A, Studitsky V. Lyubitelev A, et al. Int J Mol Sci. 2023 Jun 26;24(13):10663. doi: 10.3390/ijms241310663. Int J Mol Sci. 2023. PMID: 37445850 Free PMC article. Review.
References
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266(5192):1821–8. - PubMed
- Hirama T, Koeffler HP. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86(3):841–54. - PubMed
- Guehenneux F, Duret L, Callanan MB, Bouhas R, Hayette S, Berthet C, et al. Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia. 1997;11(3):370–5. - PubMed
- Matsuda S, Rouault J, Magaud J, Berthet C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001;497(2-3):67–72. - PubMed
- Yoshida Y, Matsuda S, Ikematsu N, Kawamura-Tsuzuku J, Inazawa J, Umemori H, et al. ANA, a novel member of Tob/BTG1 family, is expressed in the ventricular zone of the developing central nervous system. Oncogene. 1998;16(20):2687–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA111470/CA/NCI NIH HHS/United States
- R01CA111470/CA/NCI NIH HHS/United States
- T32DK007790/DK/NIDDK NIH HHS/United States
- T32 DK007790/DK/NIDDK NIH HHS/United States
- T32 DK007790-07/DK/NIDDK NIH HHS/United States
- R01 CA111470-03/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases