The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia - PubMed (original) (raw)
The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia
Roxanna A Irani et al. J Exp Med. 2009.
Abstract
Growth-restricted fetuses are at risk for a variety of lifelong medical conditions. Preeclampsia, a life-threatening hypertensive disorder of pregnancy, is associated with fetuses who suffer from intrauterine growth restriction (IUGR). Recently, emerging evidence indicates that preeclamptic women harbor AT(1) receptor agonistic autoantibodies (AT(1)-AAs) that contribute to the disease features. However, the exact role of AT(1)-AAs in IUGR and the underlying mechanisms have not been identified. We report that these autoantibodies are present in the cord blood of women with preeclampsia and retain the ability to activate AT(1) receptors. Using an autoantibody-induced animal model of preeclampsia, we show that AT(1)-AAs cross the mouse placenta, enter fetal circulation, and lead to small fetuses with organ growth retardation. AT(1)-AAs also induce apoptosis in the placentas of pregnant mice, human villous explants, and human trophoblast cells. Finally, autoantibody-induced IUGR and placental apoptosis are diminished by either losartan or an autoantibody-neutralizing peptide. Thus, these studies identify AT(1)-AA as a novel causative factor of preeclampsia-associated IUGR and offer two possible underlying mechanisms: a direct detrimental effect on fetal development by crossing the placenta and entering fetal circulation, and indirectly through AT(1)-AA-induced placental damage. Our findings highlight AT(1)-AAs as important therapeutic targets.
Figures
Figure 1.
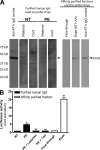
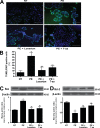
AT1-AAs can pass through the human placenta and retain biological activity in fetal circulation. (A and B) Autoantibodies detected by Western blot in the sera of preeclamptic women could also be found in cord blood (A). Cellular lysate from CHO.AT1A cells containing stably integrated copies of a minigene encoding the AT1 receptor was run on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The AT1 receptor–rich membrane was cut into strips and each strip was individually probed by either an anti–AT1 receptor antibody (control), or purified antibodies derived from maternal serum or cord blood from normotensive (n = 6) or preeclamptic (n = 6) pregnancies. Also, to specifically detect AT1-AAs from the total IgG pool, an affinity purification strategy was used. The flow-through and eluted affinity-purified fractions of IgGs derived from the cord blood of babies from preeclamptic patients were also tested for their ability to bind to the AT1 receptor (n = 6). Only preeclamptic maternal sera, cord blood from babies of preeclamptic women, and the eluted affinity-purified fraction harbored autoantibodies recognizing the AT1 receptor at 43 kD. Then, cord blood IgGs were tested for biological activity (B) using an in vitro luciferase activity assay that is increased secondary to AT1 receptor activation. Only IgGs purified from the cord blood of babies from preeclamptic patients or the eluted affinity-purified fraction induced luciferase activity. This bioactivity could be blocked by co-culturing the reporter cell line with IgG derived from preeclamptic patients and losartan or the 7-aa epitope peptide (n = 5 for each variable in two independent experiments). Data are expressed as means ± SEM. *, P < 0.01 versus IgG derived from cord blood of a normotensive patient; **, P < 0.01 versus IgG derived from cord blood of a preeclamptic patient; +, P < 0.01 versus flow-through affinity-purified fraction. NT, normotensive; PE, preeclampsia.
Figure 2.
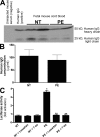
Human IgG crosses the mouse placenta and can be detected in fetal mouse sera where it retains biological activity. (A–C) Human IgG could be detected in fetal mouse circulation by Western blotting (A) and ELISA (B). IgGs from normotensive or preeclamptic pregnant women were injected into pregnant mice at E13 and E14. Fetal sera were collected upon sacrifice on E18. Human IgG was detected in fetal mouse circulation (born to dams injected with human IgG) by Western blotting (A) using an anti–human IgG antibody, but not in the circulation of mice born to dams without human IgG injection. The concentration of human IgG in fetal mouse circulation was detected by an ELISA specific for human IgG (B). IgGs from both normotensive and preeclamptic pregnancies were detectable in fetal mice circulation, and the level of IgGs in fetal circulation between the two injected groups (n = 7 for each group in three independent experiments) was not significantly different (P > 0.05). However, the only group whose serum harbored antibodies that recognized the AT1 receptor and maintained the biological activity was the fetuses of preeclamptic IgG-injected mice (C). This was assessed by using a bioassay wherein AT1 receptor activation induces luciferase activity in transfected CHO-NFAT cells (n = 5 for each variable in two independent experiments). Data are expressed as means ± SEM. *, P < 0.01 versus normotensive IgG treatment; **, P < 0.01 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
Figure 3.
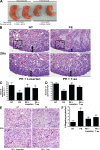
AT1 receptor activation results in decreased fetal weight and impaired organ development. AT1 receptor activation results in fetal abnormalities. (A) Injection of pregnant mice with IgGs from preeclamptic women resulted in fetuses weighing less than those born to pregnant mice injected with IgG derived from normotensive women. Only pups born in litters of six to eight were analyzed, and those depicted in the figure were selected from the center of the uterine horn (preeclamptic fetuses, n = 89; and normotensive fetuses, n = 80, collected and analyzed in four independent experiments). *, P < 0.05 versus normotensive IgG treatment. (B) Fetal mouse kidney histology (H&E staining). Bars, 50 µm. (C and D) As compared with the fetal kidneys from normotensive IgG injection litters, the kidneys from litters of preeclamptic IgG injection dams demonstrated a narrow nephrogenic zone (C) and a decreased number of glomeruli (D). The double arrows in B demarcate the nephrogenic zone. Arrowheads within the boxes indicate a glomerulus within a representative high power field. (E) Fetal mouse liver histology (H&E staining). Bars, 50 µm. (F) Compared with the livers of pups born to dams injected with normotensive IgG, the livers from pups of preeclamptic IgG injection dams showed an increased number of megakaryocytes. Arrows indicate megakaryocytes. When losartan (PE + Losartan) or the 7-aa peptide (PE + 7-aa) were coinjected into the dams, the fetal kidney and liver alterations were partially abolished (n = 12 for each variable for the histological analysis in four independent experiments). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. hpf, high power field; NT, normotensive; PE, preeclampsia.
Figure 4.
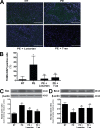
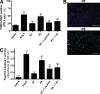
Mouse placentas demonstrate increased apoptosis caused by AT1 receptor activation. The placentas of AT1-AA–injected pregnant mice have increased apoptosis. (A) TUNEL-stained mouse placental sections demonstrate increased apoptosis in the labyrinth zone of mice injected with IgG from preeclamptic women as compared with normotensive IgG-injected mice (green, TUNEL+; blue, DAPI nuclear stain). Bars, 500 µm. (B) This qualitative increase in apoptosis is quantified and corroborated with an increased apoptotic index (percentage of TUNEL-/DAPI-positive cells) as measured in the same mouse placental sections (n = 12 placentas for each variable collected over four independent experiments). (C and D) Western blot analysis of mouse placentas indicates that AT1 receptor activation leads to increased Bax (C) and decreased Bcl-2 (D; n = 6 for each variable collected over four independent experiments). Mice coinjected with IgG from preeclamptic women and either losartan or 7-aa epitope peptide have placentas that demonstrate less apoptotic features. Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
Figure 5.
Human villous explants exhibit increased apoptosis caused by AT1 receptor activation. AT1 receptor activation induces human placental apoptosis. (A) Human villous explants cultured with IgG derived from preeclamptic patients demonstrate increased apoptosis as compared with explants incubated with IgG derived from normotensive sera. TUNEL-stained cultured human villous explants indicate that AT1-AAs, through AT1-receptor activation, increase apoptosis (green, TUNEL+; blue, DAPI nuclear stain). Bars, 500 µm. (B) Quantification of the increased apoptosis is reflected in an increased apoptotic index in the explants incubated with PE IgG (B). (C and D) Western blot analysis of explant proteins demonstrate increased Bax (C) and decreased Bcl-2 (D), indicating a proapoptotic state. Coincubation of the explants with preeclamptic IgG and losartan or 7-aa epitope peptide partially attenuated the increase in cell death. Explants of placentas from four different patients were cultured, and each variable was examined six times per placenta (n = 24). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
Figure 6.
AT1 receptor activation increases trophoblast cell apoptosis. AT1 receptor activation induces apoptosis in HTR-8/SVneo cells, a human trophoblast cell line. (A–C) HTR-8/SVneo cells cultured with IgG derived from preeclamptic patients demonstrate increased apoptosis as compared with explants incubated with IgG derived from normotensive sera. A cell death index (A) based on TUNEL-stained HTR-8/SVneo cells (B) indicates that AT1 receptor activation increases apoptosis. Caspase 3 activity (C) is also increased in HTR-8/SVneo cells cultured with IgG from preeclamptic patients as compared with those cultured with IgG from normotensive patients. Coincubation of PE IgG with losartan or 7-aa epitope peptide reduces the amount of apoptosis as well as caspase 3 activity (green, TUNEL+; blue, DAPI nuclear stain; n = 12 for each variable in three independent experiments). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia. Bars, 500 µm.
Figure 7.
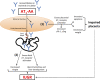
Schematic of the working model of AT1 receptor–mediated apoptosis: placental and fetal consequences. AT1 receptor activation via AT1-AAs, found in the serum of preeclamptic women, leads to (1) placental damage (2) and fetal abnormalities. sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase 1.
Similar articles
- Autoantibodies isolated from patients with preeclampsia induce soluble endoglin production from trophoblast cells via interactions with angiotensin II type 1 receptor.
Kobayashi Y, Yamamoto T, Chishima F, Takahashi H, Suzuki M. Kobayashi Y, et al. Am J Reprod Immunol. 2015 Apr;73(4):285-91. doi: 10.1111/aji.12340. Epub 2014 Nov 7. Am J Reprod Immunol. 2015. PMID: 25376533 - Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond.
Xia Y, Kellems RE. Xia Y, et al. Circ Res. 2013 Jun 21;113(1):78-87. doi: 10.1161/CIRCRESAHA.113.300752. Circ Res. 2013. PMID: 23788505 Free PMC article. Review. - Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature.
Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Walther T, et al. Hypertension. 2005 Dec;46(6):1275-9. doi: 10.1161/01.HYP.0000190040.66563.04. Epub 2005 Oct 31. Hypertension. 2005. PMID: 16260641 - Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia.
Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Zhou CC, et al. Circulation. 2010 Jan 26;121(3):436-44. doi: 10.1161/CIRCULATIONAHA.109.902890. Epub 2010 Jan 11. Circulation. 2010. PMID: 20065159 Free PMC article. - From mother to child--transplacental effect of AT1R-AA in preeclampsia.
Dragun D, Philippe A. Dragun D, et al. Nephrol Dial Transplant. 2010 Jun;25(6):1774-6. doi: 10.1093/ndt/gfq167. Epub 2010 Mar 25. Nephrol Dial Transplant. 2010. PMID: 20339097 Review. No abstract available.
Cited by
- Renin-angiotensin system in pre-eclampsia: everything old is new again.
J Spaan J, A Brown M. J Spaan J, et al. Obstet Med. 2012 Dec;5(4):147-153. doi: 10.1258/om.2012.120007. Epub 2012 Dec 6. Obstet Med. 2012. PMID: 30705695 Free PMC article. Review. - Maternal erythrocyte ENT1-mediated AMPK activation counteracts placental hypoxia and supports fetal growth.
Sayama S, Song A, Brown BC, Couturier J, Cai X, Xu P, Chen C, Zheng Y, Iriyama T, Sibai B, Longo M, Kellems RE, D'Alessandro A, Xia Y. Sayama S, et al. JCI Insight. 2020 May 21;5(10):e130205. doi: 10.1172/jci.insight.130205. JCI Insight. 2020. PMID: 32434995 Free PMC article. - Targeted expression of Cre recombinase provokes placental-specific DNA recombination in transgenic mice.
Zhou CC, Chang J, Mi T, Abbasi S, Gu D, Huang L, Zhang W, Kellems RE, Schwartz RJ, Xia Y. Zhou CC, et al. PLoS One. 2012;7(2):e29236. doi: 10.1371/journal.pone.0029236. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363401 Free PMC article. - Prenatal Hypoxia Affects Foetal Cardiovascular Regulatory Mechanisms in a Sex- and Circadian-Dependent Manner: A Review.
Sutovska H, Babarikova K, Zeman M, Molcan L. Sutovska H, et al. Int J Mol Sci. 2022 Mar 7;23(5):2885. doi: 10.3390/ijms23052885. Int J Mol Sci. 2022. PMID: 35270026 Free PMC article. Review. - Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia.
Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Zhou CC, et al. J Immunol. 2011 May 15;186(10):6024-34. doi: 10.4049/jimmunol.1004026. Epub 2011 Apr 11. J Immunol. 2011. PMID: 21482739 Free PMC article.
References
- Alexander B.T. 2003. Intrauterine growth restriction and reduced glomerular number: role of apoptosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285:R933–R934 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 HD034130/HD/NICHD NIH HHS/United States
- HD34130/HD/NICHD NIH HHS/United States
- HL076558/HL/NHLBI NIH HHS/United States
- R01 HD034130/HD/NICHD NIH HHS/United States
- R01 HL076558/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials