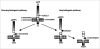Prion protein and Alzheimer disease - PubMed (original) (raw)
Review
Prion protein and Alzheimer disease
Katherine A B Kellett et al. Prion. 2009 Oct-Dec.
Abstract
Alzheimer and prion diseases are neurodegenerative disorders characterised by the abnormal processing of amyloid-beta (Abeta) peptide and prion protein (PrP(C)), respectively. Recent evidence indicates that PrP(C) may play a critical role in the pathogenesis of Alzheimer disease. PrP(C) interacts with and inhibits the beta-secretase BACE1, the rate-limiting enzyme in the production of Abeta. More recently PrP(C) was identified as a receptor for Abeta oligomers and the expression of PrP(C) appears to be controlled by the amyloid intracellular domain (AICD). Here we review these observations and propose a feedback loop in the normal brain where PrP(C) exerts an inhibitory effect on BACE1 to decrease both Abeta and AICD production. In turn, the AICD upregulates PrP(C) expression, thus maintaining the inhibitory effect of PrP(C) on BACE1. In Alzheimer disease, this feedback loop is disrupted, and the increased level of Abeta oligomers bind to PrP(C) and prevent it from regulating BACE1 activity.
Figures
Figure 1
Proteolytic processing of APP. The amyloidogenic pathway involves the sequential cleavage of APP first by BACE1, producing sAPPβ and C99, and then by γ-secretase to release the amyloidogenic Aβ and the AICD. In the non-amyloidogenic pathway, α-secretase cleaves within the Aβ fragment, preventing the formation of Aβ peptides.
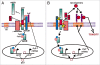
Figure 2
A model for the regulation of APP processing by PrPC. (A) Aβ levels are kept in balance under physiological conditions via inhibition of BACE1 by PrPC and regulation of PrPC levels via AICD. (B) In AD, Aβ levels are increased as a result of increased production and/or decreased degradation and clearance resulting in the increased formation of Aβ-oligomers which are toxic via their interaction with PrPC. The binding of the Aβ-oligomers to PrPC may disrupt its regulation of BACE1, thereby further increasing APP processing and Aβ levels.
Similar articles
- High molecular mass assemblies of amyloid-β oligomers bind prion protein in patients with Alzheimer's disease.
Dohler F, Sepulveda-Falla D, Krasemann S, Altmeppen H, Schlüter H, Hildebrand D, Zerr I, Matschke J, Glatzel M. Dohler F, et al. Brain. 2014 Mar;137(Pt 3):873-86. doi: 10.1093/brain/awt375. Epub 2014 Feb 10. Brain. 2014. PMID: 24519981 - Unaltered prion protein expression in Alzheimer disease patients.
Saijo E, Scheff SW, Telling GC. Saijo E, et al. Prion. 2011 Apr-Jun;5(2):109-16. doi: 10.4161/pri.5.2.16355. Epub 2011 Apr 1. Prion. 2011. PMID: 21654203 Free PMC article. - Prion protein interacts with BACE1 protein and differentially regulates its activity toward wild type and Swedish mutant amyloid precursor protein.
Griffiths HH, Whitehouse IJ, Baybutt H, Brown D, Kellett KA, Jackson CD, Turner AJ, Piccardo P, Manson JC, Hooper NM. Griffiths HH, et al. J Biol Chem. 2011 Sep 23;286(38):33489-500. doi: 10.1074/jbc.M111.278556. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795680 Free PMC article. - Amyloid-β induced signaling by cellular prion protein and Fyn kinase in Alzheimer disease.
Um JW, Strittmatter SM. Um JW, et al. Prion. 2013 Jan-Feb;7(1):37-41. doi: 10.4161/pri.22212. Epub 2012 Sep 17. Prion. 2013. PMID: 22987042 Free PMC article. Review. - The pathogenesis of soluble PrP fragments containing Aβ binding sites.
Li B. Li B. Virus Res. 2016 Jan 4;211:194-8. doi: 10.1016/j.virusres.2015.10.023. Epub 2015 Oct 31. Virus Res. 2016. PMID: 26528810 Review.
Cited by
- Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer's Disease.
Song Z, Xu Y, Deng W, Zhang L, Zhu H, Yu P, Qu Y, Zhao W, Han Y, Qin C. Song Z, et al. Front Mol Neurosci. 2020 May 29;13:79. doi: 10.3389/fnmol.2020.00079. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32547364 Free PMC article. Review. - Cellular prion protein expression is not regulated by the Alzheimer's amyloid precursor protein intracellular domain.
Lewis V, Whitehouse IJ, Baybutt H, Manson JC, Collins SJ, Hooper NM. Lewis V, et al. PLoS One. 2012;7(2):e31754. doi: 10.1371/journal.pone.0031754. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363722 Free PMC article. - PrPC Promotes Endometriosis Progression by Reprogramming Cholesterol Metabolism and Estrogen Biosynthesis of Endometrial Stromal Cells through PPARα Pathway.
Peng HY, Lei ST, Hou SH, Weng LC, Yuan Q, Li MQ, Zhao D. Peng HY, et al. Int J Biol Sci. 2022 Feb 7;18(4):1755-1772. doi: 10.7150/ijbs.68015. eCollection 2022. Int J Biol Sci. 2022. PMID: 35280685 Free PMC article. - Immunotherapy in prion disease.
Roettger Y, Du Y, Bacher M, Zerr I, Dodel R, Bach JP. Roettger Y, et al. Nat Rev Neurol. 2013 Feb;9(2):98-105. doi: 10.1038/nrneurol.2012.258. Epub 2012 Dec 18. Nat Rev Neurol. 2013. PMID: 23247613 Review. - Predicting brain-regional gene regulatory networks from multi-omics for Alzheimer's disease phenotypes and Covid-19 severity.
Khullar S, Wang D. Khullar S, et al. Hum Mol Genet. 2023 May 18;32(11):1797-1813. doi: 10.1093/hmg/ddad009. Hum Mol Genet. 2023. PMID: 36648426 Free PMC article.
References
- Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–784. - PubMed
- Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:467–471. - PubMed
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid [beta]-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. - PubMed
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials