Gas6 and the receptor tyrosine kinase Axl in clear cell renal cell carcinoma - PubMed (original) (raw)
Gas6 and the receptor tyrosine kinase Axl in clear cell renal cell carcinoma
Anna Gustafsson et al. PLoS One. 2009.
Abstract
Background: The molecular biology of renal cell carcinoma (RCC) is complex and not fully understood. We have recently found that the expression of the receptor tyrosine kinase Axl in the RCC tumors independently correlates with survival of the patients.
Principal findings: Here, we have investigated the role of Axl and its ligand Gas6, the vitamin-K dependent protein product of the growth arrest-specific gene 6, in clear cell RCC (ccRCC) derived cells. The Axl protein was highly expressed in ccRCC cells deficient in functional von Hippel-Lindau (VHL) protein, a tumor suppressor gene often inactivated in ccRCC. VHL reconstituted cells expressed decreased levels of Axl protein, but not Axl mRNA, suggesting VHL to regulate Axl expression. Gas6-mediated activation of Axl in ccRCC cells resulted in Axl phosphorylation, receptor down-regulation, decreased cell-viability and migratory capacity. No effects of the Gas6/Axl system could be detected on invasion. Moreover, in ccRCC tumor tissues, Axl was phosphorylated and Gas6 gamma-carboxylated, suggesting these molecules to be active in vivo.
Significance: These results provide novel information regarding the complex function of the Gas6/Axl system in ccRCC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
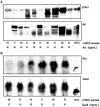
Figure 1. Axl is phosphorylated and Gas6 is γ-carboxylated in human ccRCC tissue.
Homogenized tissue lysates from a panel (patient one to eight) of matched ccRCC tissues and unaffected kidney cortex counterparts were used for Axl (A) and Gas6 (B) immunoprecipitation with specific polyclonal antisera (#R042 towards Axl N-terminal; #005 towards Gas6) containing 50–100 µg total immunoglobulin molecules (about 0.5–1.0 µg specific immunoprecipitating antibody). Pellets were separated on a 6% denaturing SDS-PAGE gel. Protein expression and posttranslational modifications were verified with western blot analysis using Axl (Axl-C20; towards Axl C-terminal), Gas6 (R&D), phosphotyrosine (PY99) and Gla (M3b; 5 mM EDTA final) specific antibodies, respectively. Membranes were first immunoblotted for phosphotyrosine and Gla, respectively, and thereafter stripped and reprobed for total Axl and Gas6. For each patient the matched samples are denoted: N (normal unaffected kidney cortex) and T (ccRCC tissue). Absolute concentrations in the lysates measured by ELISA are depicted below each sample.
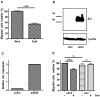
Figure 2. Axl but not Gas6 is expressed in ccRCC 786-O cells and can be functionally active.
Expression of Axl (A) and Gas6 (B) were analyzed by western blot analysis using total cell lysate (tcl; Axl) and immunoprecipitate (Gas6) separated on an 8% reducing SDS-PAGE gel, and using Axl (Axl-C20) and Gas6 (R&D) specific antibodies, respectively. Gas6 expression in 786-O cells, grown in conditioned medium with and without vitamin K, was compared to expression in stably transfected Hek293 cells . Gas6-dependent Axl phosphorylation was analysed in serum-starved (1% FCS; 20 h) 786-O cells stimulated with various Gas6 concentrations for 10 min (C) and stimulated with 400 ng/mL Gas6 for up to 1 h (D). Total cell lysate of stimulated cells were subjected to Axl immunoprecipitation with an in house Axl-specific polyclonal antibody (R042) and immune-complexes were thereafter separated on 8% reducing SDS-PAGE and immunoblotted for phosphorylated Axl (PY99). The membranes were stripped and reblotted for total amount of immunoprecipitated Axl (Axl-C20). Representative western blots are shown.
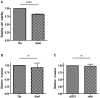
Figure 3. Gas6-dependent Axl-mediated inhibition of ccRCC 786-O cell migration.
The migratory capacity of 786-O cells upon Gas6 stimulation was analyzed using the Boyden chamber assay. (A) Gas6 stimulation (400 ng/mL) for 4 h decreases the migratory capacity of 786-O cells to about 80% in comparison to that of mock treated cells. Verification of the Axl knockdown using siAxl and siSCR treated cells by either (B) western blot analysis or (C) qRT-PCR as described in material and methods. (D) The inhibition of migration due to Gas6 stimulation was a consequence of ligand specific activation of the Axl receptor as confirmed by repeating the migration experiment using the siAxl and siSCR treated 786-O cells. Each experiment was conducted in triplicates, and the result out of three independent experiments is shown. Fig. 3_B,C_ shows results from one representative experiment.
Figure 4. Gas6-dependent Axl-mediated effects on ccRCC 786-O cell viability and invasion.
Viability of 786-O cells were analysed using the MTT assay. (A) Stimulation of 786-O cells with 400 ng/mL Gas6 for 24 h resulted in a viability of about 80% in comparison to that of control treated cells. (B) Invasion of 786-O cells using the modified Boyden chamber assay with a matrigel layer for analysis of invasive capacity was not affected by Gas6 stimulation (400 ng/mL). (C) Axl expression per se did not contribute to the high invasive phenotype of 786-O cells, since the lack of Axl expression did not result in any significant diminished invasive capacity. Each experiment was performed using at least triplicates and the result is shown from three independent experiments.
Figure 5. Gas6- and VHL-dependent Axl protein expression in ccRCC 786-O cells.
(A) Serum-starved 786-O cells (1% FCS; 20 h) were stimulated with 400 ng/mL Gas6 or mock control for up to 6 h. Gas6-dependendent Axl protein down-regulation over time were verified with western blot analysis on total cell lysate from treated cells using 8% reducing SDS-PAGE. Membranes were immunoblotted with Axl specific antibodies (Axl-C20) and reprobed with β-actin specific antibodies (A5441) for relative quantification of Axl expression levels. Each experiment was run in duplicates and the result of three independent experiments is shown. (B) Axl expression in 786-O mock (VHL defective) or VHL reconstituted cells was analyzed in cells grown in either normoxia or hypoxia (1% O2) under serum starvation (1% FCS) for 48 h. VHL-dependent Axl expression was verified using western blot analysis of total cell lysates of treated cells that were separated on an 8% reducing SDS-PAGE gel. Membranes were immunoblotted with Axl-specific antibodies (Axl-C20) and with β-actin specific antibodies (A5441) for relative quantification of Axl expression levels. The experiment was carried out using a minimum of triplicates. (C) Axl inverse correlation to functional VHL protein was further analyzed by immunoblotting for Axl expression in total cell lysates of a panel of ccRCC cell lines with different VHL status. Equal amounts of total protein were separated on an 8% reducing SDS-PAGE gel and membrane immunoblotted with Axl-specific antibodies (Axl-C20).
Similar articles
- S100A10 Is a Critical Mediator of GAS6/AXL-Induced Angiogenesis in Renal Cell Carcinoma.
Xiao Y, Zhao H, Tian L, Nolley R, Diep AN, Ernst A, Fuh KC, Miao YR, von Eyben R, Leppert JT, Brooks JD, Peehl DM, Giaccia AJ, Rankin EB. Xiao Y, et al. Cancer Res. 2019 Nov 15;79(22):5758-5768. doi: 10.1158/0008-5472.CAN-19-1366. Epub 2019 Oct 4. Cancer Res. 2019. PMID: 31585940 Free PMC article. - Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET.
Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, LaGory EL, Kariolis MS, Chan A, Lindgren D, Axelson H, Miao YR, Krieg AJ, Giaccia AJ. Rankin EB, et al. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13373-8. doi: 10.1073/pnas.1404848111. Epub 2014 Sep 3. Proc Natl Acad Sci U S A. 2014. PMID: 25187556 Free PMC article. - Gas6-Axl signaling in presence of Sunitinib is enhanced, diversified and sustained in renal tumor cells, resulting in tumor-progressive advantages.
Gustafsson A, Fritz HKM, Dahlbäck B. Gustafsson A, et al. Exp Cell Res. 2017 Jun 1;355(1):47-56. doi: 10.1016/j.yexcr.2017.03.040. Epub 2017 Mar 19. Exp Cell Res. 2017. PMID: 28327411 - Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily.
Hafizi S, Dahlbäck B. Hafizi S, et al. FEBS J. 2006 Dec;273(23):5231-44. doi: 10.1111/j.1742-4658.2006.05529.x. Epub 2006 Oct 25. FEBS J. 2006. PMID: 17064312 Review. - Therapeutic Targeting of the Gas6/Axl Signaling Pathway in Cancer.
Tanaka M, Siemann DW. Tanaka M, et al. Int J Mol Sci. 2021 Sep 15;22(18):9953. doi: 10.3390/ijms22189953. Int J Mol Sci. 2021. PMID: 34576116 Free PMC article. Review.
Cited by
- Genetic and Epigenetic Characteristics in Isolated Pancreatic Metastases of Clear-Cell Renal Cell Carcinoma.
Sellner F, Compérat E, Klimpfinger M. Sellner F, et al. Int J Mol Sci. 2023 Nov 14;24(22):16292. doi: 10.3390/ijms242216292. Int J Mol Sci. 2023. PMID: 38003482 Free PMC article. Review. - In vitro and in vivo antiangiogenic activity of desacetylvinblastine monohydrazide through inhibition of VEGFR2 and Axl pathways.
Lei X, Chen M, Nie Q, Hu J, Zhuo Z, Yiu A, Chen H, Xu N, Huang M, Ye K, Bai L, Ye W, Zhang D. Lei X, et al. Am J Cancer Res. 2016 Mar 15;6(4):843-58. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186435 Free PMC article. - S100A10 Is a Critical Mediator of GAS6/AXL-Induced Angiogenesis in Renal Cell Carcinoma.
Xiao Y, Zhao H, Tian L, Nolley R, Diep AN, Ernst A, Fuh KC, Miao YR, von Eyben R, Leppert JT, Brooks JD, Peehl DM, Giaccia AJ, Rankin EB. Xiao Y, et al. Cancer Res. 2019 Nov 15;79(22):5758-5768. doi: 10.1158/0008-5472.CAN-19-1366. Epub 2019 Oct 4. Cancer Res. 2019. PMID: 31585940 Free PMC article. - Modulation of protein S and growth arrest specific 6 protein signaling inhibits pancreatic cancer cell survival and proliferation.
Pilli VS, Datta A, Dorsey A, Liu B, Majumder R. Pilli VS, et al. Oncol Rep. 2020 Oct;44(4):1322-1332. doi: 10.3892/or.2020.7689. Epub 2020 Jul 15. Oncol Rep. 2020. PMID: 32945517 Free PMC article.
References
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. - PubMed
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–57. - PubMed
- Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. - PubMed
- Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. Febs J. 2006;273:5231–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous