Brain cancer propagating cells: biology, genetics and targeted therapies - PubMed (original) (raw)
Review
Brain cancer propagating cells: biology, genetics and targeted therapies
Costas G Hadjipanayis et al. Trends Mol Med. 2009 Nov.
Abstract
Cancer propagating cells (CPCs) within primary central nervous system (CNS) tumors (glioblastoma multiforme (GBM), medulloblastoma (MB) and ependymoma) might be integral to tumor development and perpetuation. These cells, also known as brain cancer propagating cells (BCPCs), have the ability to self-renew and proliferate. BCPCs can initiate new tumors in mice with high efficiency and these exhibit many features that are characteristic of patient's brain tumors. Accumulating evidence suggests that BCPCs might originate from the transformation of neural stem cells (NSCs) and their progenitors. Furthermore, recent studies have shown that NSC surface markers also define BCPCs. Ultimately, treatments that include specific targeting of BCPCs might potentially be more effective at treating the entire tumor mass, translating to improved patient survival and quality of life.
Figures
Figure I
Figure I
Photographs courtesy of D. Brat and C. Tucker-Burden.
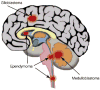
Figure 1
Brain tumors. Location of GBM, MB and ependymoma tumors in the CNS.
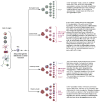
Figure 2
Normal CNS differentiation and transformation for CNS tumor formation. NSCs can differentiate into neural and glial progenitors. Neural progenitors differentiate into neurons, whereas glial progenitors are committed to oligodendrocytes, ependymal cells or astrocytes. CNS tumor formation might originate from the transformation of NSCs into BCPCs. Glial progenitors might transform into brain tumor progenitor-like cells that could engender CNS tumors (GBMs, MBs and ependymomas). The transformation of neurons, oligodendrocytes, ependymal cells and astrocytes has traditionally been thought to form CNS tumors. BCPCs can differentiate into brain tumor progenitor-like cells or more differentiated progeny, which can lead to CNS tumor formation. Stromal cells, either from the local brain microenvironment or recruited systemically, can be essential for tumor maintenance, progression and recurrence.
Figure 3
Human neurospheres cultured from glioblastoma tumor. The figure shows different magnifications of cultured neurospheres (4×, 20× and 40×).
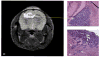
Figure 4
Human glioblastoma xenograft generated in an athymic nu/nu mouse eight weeks after the intracranial implantation of neurospheres harvested from a resected glioblastoma specimen. a) T2-weighted MRI of mouse brain showing xenograft (marked by *). b) Hematoxylin/eosin stained section of mouse brain showing xenograft (marked by arrow). c) Hematoxylin/eosin stained section of mouse brain demonstrating the invasion of human glioblastoma xenograft into surrounding brain (marked by arrows).
Similar articles
- [Progress in the study of brain tumor stem cells as treatment targets].
Hide T, Kuratsu J. Hide T, et al. Brain Nerve. 2009 Jul;61(7):781-9. Brain Nerve. 2009. PMID: 19618855 Review. Japanese. - Cancer stem cells in pediatric brain tumors.
Lasky JL 3rd, Choe M, Nakano I. Lasky JL 3rd, et al. Curr Stem Cell Res Ther. 2009 Dec;4(4):298-305. doi: 10.2174/157488809789649278. Curr Stem Cell Res Ther. 2009. PMID: 19500067 Review. - Understanding the role of tumor stem cells in glioblastoma multiforme: a review article.
Fatoo A, Nanaszko MJ, Allen BB, Mok CL, Bukanova EN, Beyene R, Moliterno JA, Boockvar JA. Fatoo A, et al. J Neurooncol. 2011 Jul;103(3):397-408. doi: 10.1007/s11060-010-0406-3. Epub 2010 Sep 18. J Neurooncol. 2011. PMID: 20853017 Review. - Pediatric brain tumor cell lines.
Xu J, Margol A, Asgharzadeh S, Erdreich-Epstein A. Xu J, et al. J Cell Biochem. 2015 Feb;116(2):218-24. doi: 10.1002/jcb.24976. J Cell Biochem. 2015. PMID: 25211508 Free PMC article. - Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma.
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, dePinho RA. Zheng H, et al. Cold Spring Harb Symp Quant Biol. 2008;73:427-37. doi: 10.1101/sqb.2008.73.047. Epub 2009 Jan 15. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19150964
Cited by
- N-cadherin upregulation mediates adaptive radioresistance in glioblastoma.
Osuka S, Zhu D, Zhang Z, Li C, Stackhouse CT, Sampetrean O, Olson JJ, Gillespie GY, Saya H, Willey CD, Van Meir EG. Osuka S, et al. J Clin Invest. 2021 Mar 15;131(6):e136098. doi: 10.1172/JCI136098. J Clin Invest. 2021. PMID: 33720050 Free PMC article. - Design and in vitro activities of N-alkyl-N-[(8-R-2,2-dimethyl-2H-chromen-6-yl)methyl]heteroarylsulfonamides, novel, small-molecule hypoxia inducible factor-1 pathway inhibitors and anticancer agents.
Mun J, Jabbar AA, Devi NS, Yin S, Wang Y, Tan C, Culver D, Snyder JP, Van Meir EG, Goodman MM. Mun J, et al. J Med Chem. 2012 Aug 9;55(15):6738-50. doi: 10.1021/jm300752n. Epub 2012 Jul 24. J Med Chem. 2012. PMID: 22746274 Free PMC article. - Interaction of hypoxia-inducible factor-1α and Notch signaling regulates medulloblastoma precursor proliferation and fate.
Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, D'Avella D, Panchision DM, Della Puppa A, Scienza R, Basso G. Pistollato F, et al. Stem Cells. 2010 Nov;28(11):1918-29. doi: 10.1002/stem.518. Stem Cells. 2010. PMID: 20827750 Free PMC article. - Human Brat ortholog TRIM3 is a tumor suppressor that regulates asymmetric cell division in glioblastoma.
Chen G, Kong J, Tucker-Burden C, Anand M, Rong Y, Rahman F, Moreno CS, Van Meir EG, Hadjipanayis CG, Brat DJ. Chen G, et al. Cancer Res. 2014 Aug 15;74(16):4536-48. doi: 10.1158/0008-5472.CAN-13-3703. Epub 2014 Jun 19. Cancer Res. 2014. PMID: 24947043 Free PMC article. - Potential lethal damage repair in glioblastoma cells irradiated with ion beams of various types and levels of linear energy transfer.
Chew MT, Nisbet A, Suzuki M, Matsufuji N, Murakami T, Jones B, Bradley DA. Chew MT, et al. J Radiat Res. 2019 Jan 1;60(1):59-68. doi: 10.1093/jrr/rry081. J Radiat Res. 2019. PMID: 30452663 Free PMC article.
References
- Jordan CT, et al. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. - PubMed
- Reya T, et al. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. - PubMed
- Taylor MD, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. - PubMed
- Singh SK, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA086335/CA/NCI NIH HHS/United States
- CA116804/CA/NCI NIH HHS/United States
- K08 NS053454-01A1/NS/NINDS NIH HHS/United States
- CA86335/CA/NCI NIH HHS/United States
- K08 NS053454/NS/NINDS NIH HHS/United States
- K08 NS053454-04/NS/NINDS NIH HHS/United States
- NS053454/NS/NINDS NIH HHS/United States
- K08 NS053454-02/NS/NINDS NIH HHS/United States
- K08 NS053454-03/NS/NINDS NIH HHS/United States
- R01 CA116804/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous