Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines - PubMed (original) (raw)
Comparative Study
Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines
Jan T Poolman et al. Clin Vaccine Immunol. 2010 Jan.
Abstract
The history of the pneumococcal polysaccharide enzyme-linked immunosorbent assay (ELISA) is characterized by a continuous search for increased specificity. A third-generation ELISA that uses 22F polysaccharide inhibition has increased the specificity of the assay, particularly at low antibody concentrations. The present work compared various 22F ELISAs and non-22F ELISAs. The comparisons involved three different laboratories, including a WHO reference laboratory, and included sera from subjects from different geographic areas immunized with different pneumococcal conjugate vaccines, including the licensed 7-valent Prevenar vaccine and the 10-valent Synflorix vaccine. All comparisons led to the same conclusion that the threshold defined as 0.35 microg/ml for the WHO non-22F ELISA is lower when any 22F ELISA is used. The use of highly purified polysaccharides for coating further improved the specificity of the assay. In conclusion, we confirm that the 22F ELISA can be recommended as a reference method for the determination of antibodies against pneumococcal polysaccharides.
Figures
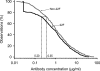
FIG. 1.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in the sera of infants from Europe and Latin America after immunization with a pneumococcal conjugate vaccine for comparison of the GSK 22F ELISA and the WHO non-22F ELISA (study A). Aggregate reverse cumulative distribution curves were plotted by using the concentrations of antibodies against pneumococcal serotype 4, 6B, 9V, 14, 18C, 19F, and 23F polysaccharides.
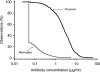
FIG. 2.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with the Prevenar or the Meningitec vaccine by using the GSK 22F ELISA (study B).
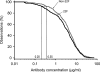
FIG. 3.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with the Prevenar vaccine for comparison of the WHO non-22F ELISA and the WHO 22F ELISA, both of which were performed at ICH, with the GSK 22F ELISA, which was performed at GSK (study C). In addition to our standard comparison of the WHO non-22F ELISA (threshold, 0.35 μg/ml) with the two 22F ELISAs (GSK 22 [dotted lines] and WHO 22F [blue dashed line]), a comparison of the WHO 22F ELISA (by use of a 0.35-μg/ml threshold) and the GSK 22F ELISA was also performed (red dashed line).
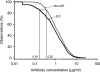
FIG. 4.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations measured in pediatric sera after immunization with a nine-valent pneumococcal conjugate vaccine for comparison of the THL non-22F ELISA and 22F ELISA (study D) by using the concentration of antibodies against pneumococcal serotypes 1, 6B, 14, 19F, and 23F. Data for two groups of vaccinated children, HIV-infected children (n = 20) and non-HIV-infected children (n = 58), were pooled. The data from both groups combined were used to prepare the RCDCs.
FIG. 5.
Aggregate reverse cumulative distribution curves of anti-PS IgG concentrations in pediatric sera measured after immunization with the Synflorix or the Prevenar vaccine for comparison of the WHO non-22F ELISA and the GSK 22F ELISA, both of which were performed at the GSK laboratory (study E).
Similar articles
- Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera.
Henckaerts I, Goldblatt D, Ashton L, Poolman J. Henckaerts I, et al. Clin Vaccine Immunol. 2006 Mar;13(3):356-60. doi: 10.1128/CVI.13.3.356-360.2006. Clin Vaccine Immunol. 2006. PMID: 16522777 Free PMC article. - Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies.
Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. Siber GR, et al. Vaccine. 2007 May 10;25(19):3816-26. doi: 10.1016/j.vaccine.2007.01.119. Epub 2007 Feb 21. Vaccine. 2007. PMID: 17368878 - Evaluation and Optimization of an ELISA Procedure to Quantify Antibodies Against Pneumococcal Polysaccharides Included in the 13-Valent Conjugate Vaccine.
Belmonti S, Lombardi F, Morandi M, Fabbiani M, Tordini G, Cauda R, De Luca A, Di Giambenedetto S, Montagnani F. Belmonti S, et al. J Immunoassay Immunochem. 2016;37(2):189-200. doi: 10.1080/15321819.2015.1106949. J Immunoassay Immunochem. 2016. PMID: 26506438 - Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults.
Durando P, Faust SN, Fletcher M, Krizova P, Torres A, Welte T. Durando P, et al. Clin Microbiol Infect. 2013 Oct;19 Suppl 1:1-9. doi: 10.1111/1469-0691.12320. Clin Microbiol Infect. 2013. PMID: 24083785 Review. - Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides.
Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, Käyhty H, Jonsdottir I, Nahm MH. Wernette CM, et al. Clin Diagn Lab Immunol. 2003 Jul;10(4):514-9. doi: 10.1128/cdli.10.4.514-519.2003. Clin Diagn Lab Immunol. 2003. PMID: 12853378 Free PMC article. Review. No abstract available.
Cited by
- Reactogenicity, safety and immunogenicity of a protein-based pneumococcal vaccine in Gambian children aged 2-4 years: A phase II randomized study.
Odutola A, Ota MO, Ogundare EO, Antonio M, Owiafe P, Worwui A, Greenwood B, Alderson M, Traskine M, Verlant V, Dobbelaere K, Borys D. Odutola A, et al. Hum Vaccin Immunother. 2016;12(2):393-402. doi: 10.1080/21645515.2015.1111496. Hum Vaccin Immunother. 2016. PMID: 26618243 Free PMC article. Clinical Trial. - Safety and immunogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Nigerian children: Booster dose and 2-dose catch-up regimens in the second year of life.
Odusanya OO, Kuyinu YA, Kehinde OA, Shafi F, François N, Yarzabal JP, Dobbelaere K, Rüggeberg JU, Borys D, Schuerman L. Odusanya OO, et al. Hum Vaccin Immunother. 2014;10(3):757-66. doi: 10.4161/hv.27276. Epub 2013 Dec 4. Hum Vaccin Immunother. 2014. PMID: 24356787 Free PMC article. - Immune Responses to pneumococcal vaccines in children and adults: Rationale for age-specific vaccination.
Westerink MA, Schroeder HW Jr, Nahm MH. Westerink MA, et al. Aging Dis. 2012 Feb;3(1):51-67. Epub 2011 Jul 20. Aging Dis. 2012. PMID: 22500271 Free PMC article. - Rapid kinetics of serum IgA after vaccination with Prevnar®13 followed by Pneumovax®23.
Crowther RR, Collins CM, Conley C, Lopez OJ. Crowther RR, et al. Heliyon. 2017 Feb 22;3(2):e00255. doi: 10.1016/j.heliyon.2017.e00255. eCollection 2017 Feb. Heliyon. 2017. PMID: 28275739 Free PMC article. - Safety, reactogenicity and immunogenicity of a booster dose of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Malian children.
Dicko A, Santara G, Mahamar A, Sidibe Y, Barry A, Dicko Y, Diallo A, Dolo A, Doumbo O, Shafi F, François N, Strezova A, Borys D, Schuerman L. Dicko A, et al. Hum Vaccin Immunother. 2013 Feb;9(2):382-8. doi: 10.4161/hv.22692. Epub 2013 Jan 4. Hum Vaccin Immunother. 2013. PMID: 23291945 Free PMC article. Clinical Trial.
References
- Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
- Feavers, I., I. Knezevic, M. Powell, and E. Griffiths. 2009. Challenges in the evaluation and licensing of new pneumococcal vaccines, 7-8 July 2008, Ottawa, Canada. Vaccine 27:3681-3688. - PubMed
- Fischer, W., T. Behr, R. Hartmann, J. Peter-Katalinic, and H. Egge. 1993. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur. J. Biochem. 215:851-857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources