Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle - PubMed (original) (raw)
Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle
Wenju Lu et al. Am J Physiol Cell Physiol. 2010 Jan.
Abstract
In pulmonary arterial smooth muscle cells (PASMCs), Ca2+ influx through store-operated Ca2+ channels thought to be composed of canonical transient receptor potential (TRPC) proteins is an important determinant of intracellular free calcium concentration ([Ca2+](i)) and pulmonary vascular tone. Sildenafil, a type V phosphodiesterase inhibitor that increases cellular cGMP, is recently identified as a promising agent for treatment of pulmonary hypertension. We previously demonstrated that chronic hypoxia elevated basal [Ca2+](i) in PASMCs due in large part to enhanced store-operated Ca2+ entry (SOCE); moreover, ex vivo exposure to prolonged hypoxia (4% O2 for 60 h) upregulated TRPC1 and TRPC6 expression in PASMCs. We examined the effect of sildenafil on basal [Ca2+](i), SOCE, and the expression of TRPC in PASMCs under prolonged hypoxia exposure. We also examined the effect of sildenafil on TRPC1 and TRPC6 expression in pulmonary arterial smooth muscle (PA) from rats that developed chronically hypoxic pulmonary hypertension (CHPH). Compared with vehicle control, treatment with sildenafil (300 nM) inhibited prolonged hypoxia induced increases of 1) basal [Ca2+](i), 2) SOCE, and 3) mRNA and protein expression of TRPC in PASMCs. Moreover, sildenafil (50 mg . kg(-1) . day(-1)) inhibited mRNA and protein expression of TRPC1 and TRPC6 in PA from chronically hypoxic (10% O2 for 21 days) rats, which was associated with decreased right ventricular pressure and right ventricular hypertrophy. Furthermore, we found, in PASMCs exposed to prolonged hypoxia, that knockdown of TRPC1 or TRPC6 by their specific small interference RNA attenuated the hypoxic increases of SOCE and basal [Ca2+]i, suggesting a cause and effect link between increases of TRPC1 and TRPC6 expression and the hypoxic increases of SOCE and basal [Ca2+]i. These results suggest that sildenafil may alter basal [Ca2+](i) in PASMCs by decreasing SOCE through downregulation of TRPC1 and TRPC6 expression, thereby contributing to decreased vascular tone of pulmonary arteries during the development of CHPH.
Figures
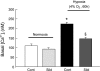
Fig. 1.
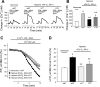
Changes in basal intracellular free calcium concentration ([Ca2+]i) in pulmonary arterial smooth muscle cells (PASMCs) treated with normoxia control (n = 7 experiments in 205 cells), normoxia plus sildenafil (n = 7 experiments in 196 cells), hypoxia control (4% O2; n = 7 experiments in 210 cells), or hypoxia plus sildenafil (n = 7 experiments in 198 cells) for 60 h. Bar values are means ± SE. *P < 0.001 vs. normoxic control cells and §P < 0.01 vs. hypoxic control cells. Cont, control; Sild, sildenafil.
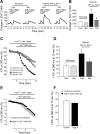
Fig. 2.
Store-operated Ca2+ entry (SOCE) in PASMCs assessed by Ca2+ restoration (A and B) and Mn2+ quenching (C_–_F). A: representative traces of [Ca2+]i responses to restoration of extracellular [Ca2+] to 2.5 mM after perfusion with Ca2+-free Krebs-Ringer bicarbonate solution (KRBS; 0 Ca2+) containing cyclopiazonic acid (CPA; 10 μM), nifedipine (Nifed, 5 μM), and EGTA (1 mM) in PASMCs treated with normoxia control (n = 4 experiments in 115 cells), normoxia plus sildenafil (n = 4 experiments in 116 cells), hypoxia control (4% O2; n = 5 experiments in 146 cells), or hypoxia plus sildenafil (n = 5 experiments in 145 cells) for 60 h. Note that the initial transient increase in [Ca2+]i after restoration of extracellular [Ca2+] was not different among the groups. B: average maximum increase in [Ca2+]i after restoration of extracellular [Ca2+]. *P < 0.001 vs. normoxia control and §P < 0.05 vs. hypoxia control. C: time course of Fura-2 fluorescence excited at 360 nm before and after addition of 200 μM Mn2+ in Ca2+-free KRBS (0 Ca2+) perfusates containing 10 μM CPA and 5 μM Nifed in PASMCs treated with normoxia control (n = 3 experiments in 82 cells), normoxia plus sildenafil (n = 3 experiments in 85 cells), hypoxia control (4% O2; n = 4 experiments in 110 cells), or hypoxia plus sildenafil (n = 4 experiments in 102 cells) for 60 h. Data were normalized to fluorescence at time 0 (F/F0). D: average quenching of Fura-2 fluorescence by Mn2+ shown in C. Data are expressed as the percentage decrease in fluorescence at time 10 min from time 0. *P < 0.05 vs. normoxia control and §P < 0.05 vs. hypoxia control. E: time course of Fura-2 fluorescence excited at 360 nM before and after addition of 200 μM Mn2+ in Ca2+-free KRBS (0 Ca2+) perfusates containing 10 μM CPA and 5 μM Nifed with (high K, with 122.7 mM KCl) or without KCl substitution (control, with 4.7 mM KCl) in PASMCs. Data were normalized to fluorescence at time 0 (F/F0). F: average quenching of Fura-2 fluorescence by Mn2+ shown in E. Data are expressed as the percentage decrease in fluorescence at time 10 min from time 0; n = 3 in each group. All bar values are means ± SE.
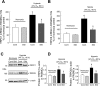
Fig. 3.
A and B: expression of canonical transient receptor potential 1 (TRPC1; A) and 6 (TRPC6; B) mRNA relative to 18s in PASMCs treated with sildenafil (300 nM) or vehicle (control) for 60 h under normoxic or hypoxic (4% O2) conditions as determined by real-time quantitative PCR and expressed as percentage of normoxia control. C and D: expression of TRPC1 and TRPC6 protein relative to α-actin in PASMCs treated with sildenafil (300 nM) or vehicle (control) for 60 h under normoxic or hypoxic (4% O2) conditions as determined by Western blotting. C: representative blots for TRPC1, TRPC6, and α-actin. D: mean protein expression for TRPC1 and TRPC6 relative to α-actin. Bar values are means ± SE; n = 4 in each group. *P < 0.01 vs. respective normoxia control and §P < 0.01 vs. respective hypoxia control.
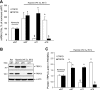
Fig. 4.
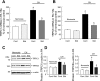
A: expression of TRPC1 and TRPC6 mRNA relative to 18s in PASMCs treated with nontargeting control small interfering RNA (siNT), siRNA targeted to TRPC1 (siT1), or siRNA targeted to TRPC6 (siT6) for 60 h under normoxic or hypoxic (4% O2) conditions as measured by real-time quantitative PCR and expressed as percentage of normoxic siNT control. B and C: expression of TRPC1 and TRPC6 protein relative to α-actin as detected by Western blotting in PASMCs treated with siNT, siT1, or siT6 for 60 h under normoxic or hypoxic (4% O2) conditions. B: representative blots for TRPC1, TRPC6, and α-actin. Nor, normoxia. C: mean protein expression for TRPC1 and TRPC6 relative to α-actin, expressed as fold change to normoxic siNT. Bar values are means ± SE; n = 3 in each group. *P < 0.01 vs. respective normoxic siNT and §P < 0.01 vs. respective hypoxic siNT.
Fig. 5.
Effects of TRPC1 and TRPC6 knockdown on hypoxic increases of SOCE in PASMCs as assessed by [Ca2+] restoration (A and B) and Mn2+ quenching (C and D) are shown. A: representative traces of [Ca2+]i responses to restoration of extracellular [Ca2+] to 2.5 mM after perfusion with Ca2+-free KRBS (0 Ca2+) containing 10 μM CPA, 5 μM Nifed, and 1 mM EGTA in PASMCs treated with siNT, siT1, or siT6 for 60 h under normoxic or hypoxic (4% O2) conditions. B: average maximum increase in [Ca2+]i after restoration of extracellular [Ca2+] in normoxic siNT, hypoxic siNT, siT1, or siT6 PASMCs. *P < 0.001 vs. normoxic siNT, **P < 0.01 vs. hypoxic siNT, and §P < 0.05 vs. hypoxic siT1. C: time course of Fura-2 fluorescence excited at 360 nm before and after addition of 200 μM Mn2+ in Ca2+-free KRBS (0 Ca2+) perfusates containing 10 μM CPA and 5 μM Nifed in PASMCs treated with siNT, siT1, or siT6 for 60 h under normoxic or hypoxic (4% O2) conditions. D: average quenching of Fura-2 fluorescence by Mn2+ shown in C. Data are expressed as the percentage decrease in fluorescence at time 10 min from time 0. *P < 0.01 vs. normoxic siNT, **P < 0.01 vs. hypoxic siNT, and §P < 0.05 vs. hypoxic siT1 and normoxic siNT. Bar values are means ± SE; n = 4 in each group.
Fig. 6.
Changes in basal [Ca2+]i in PASMCs treated with siNT, siT1, or siT6 for 60 h under normoxic or hypoxic (4% O2) conditions. Bar values are means ± SE; n = 4 in each group. *P < 0.001 vs. normoxic siNT, **P < 0.05 vs. both normoxic and hypoxic siNT, and §P < 0.05 vs. hypoxic siT1.
Fig. 7.
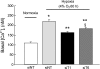
A and B: mean right ventricular pressure (RVP; A) and right ventricular systolic pressure (RVSP; B) in normoxic control rats and animals treated with normoxia plus sildenafil (50 mg · kg−1 · day−1), chronic hypoxia (CH; 10% O2 for 21 days), or CH plus sildenafil. C and D: ratio of right ventricle to left ventricle plus septum [RV/(LV + S)] (C) and ratio of right ventricle to body weight (RV/body weight) (D) in normoxic control rats and animals treated with normoxia plus sildenafil (50 mg · kg−1 · day−1), CH (10% O2 for 21 days), or CH plus sildenafil. Bar values are means ± SE; n = 7 in each group. *P < 0.01 and **P < 0.05 vs. respective normoxic control and §P < 0.01 vs. respective CH control.
Fig. 8.
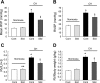
A and B: expression of TRPC1 (A) and TRPC6 (B) mRNA relative to 18s in distal pulmonary arteries (PA) from rats exposed to normoxia or CH (10% O2 for 21 days) and treated with or without sildenafil (50 mg · kg−1 · day−1) as determined by real-time quantitative PCR. C and D: expression of TRPC1 and TRPC6 protein relative to α-actin in distal PA from rats exposed to normoxia or CH (10% O2 for 21 days) and treated with or without sildenafil (50 mg · kg−1 · day−1) as determined by Western blotting. C: representative blots for TRPC1, TRPC6, and α-actin. D: mean protein expression for TRPC1 and TRPC6 relative to α-actin. Bar values are means ± SE; n = 4 in each group. *P < 0.05 vs. respective normoxia control and §P < 0.05 vs. respective CH control.
Similar articles
- Effects of chronic exposure to cigarette smoke on canonical transient receptor potential expression in rat pulmonary arterial smooth muscle.
Wang J, Chen Y, Lin C, Jia J, Tian L, Yang K, Zhao L, Lai N, Jiang Q, Sun Y, Zhong N, Ran P, Lu W. Wang J, et al. Am J Physiol Cell Physiol. 2014 Feb 15;306(4):C364-73. doi: 10.1152/ajpcell.00048.2013. Epub 2013 Dec 11. Am J Physiol Cell Physiol. 2014. PMID: 24336649 Free PMC article. - Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARγ axis.
Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, Zhang Y, Zhong N, Ran P, Lu W. Wang J, et al. Am J Respir Cell Mol Biol. 2013 Aug;49(2):231-40. doi: 10.1165/rcmb.2012-0185OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526219 Free PMC article. - Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats.
Wang J, Jiang Q, Wan L, Yang K, Zhang Y, Chen Y, Wang E, Lai N, Zhao L, Jiang H, Sun Y, Zhong N, Ran P, Lu W. Wang J, et al. Am J Respir Cell Mol Biol. 2013 Jan;48(1):125-34. doi: 10.1165/rcmb.2012-0071OC. Epub 2012 Oct 11. Am J Respir Cell Mol Biol. 2013. PMID: 23065131 Free PMC article. - New insights into the contribution of arterial NCX to the regulation of myogenic tone and blood pressure.
Zhang J. Zhang J. Adv Exp Med Biol. 2013;961:329-43. doi: 10.1007/978-1-4614-4756-6_28. Adv Exp Med Biol. 2013. PMID: 23224892 Review. - TRPC channels in smooth muscle cells.
Gonzalez-Cobos JC, Trebak M. Gonzalez-Cobos JC, et al. Front Biosci (Landmark Ed). 2010 Jun 1;15(3):1023-39. doi: 10.2741/3660. Front Biosci (Landmark Ed). 2010. PMID: 20515740 Free PMC article. Review.
Cited by
- New perspectives for the treatment of pulmonary hypertension.
Baliga RS, MacAllister RJ, Hobbs AJ. Baliga RS, et al. Br J Pharmacol. 2011 May;163(1):125-40. doi: 10.1111/j.1476-5381.2010.01164.x. Br J Pharmacol. 2011. PMID: 21175577 Free PMC article. Review. - Proteomic analysis reveals that proteasome subunit beta 6 is involved in hypoxia-induced pulmonary vascular remodeling in rats.
Wang J, Xu L, Yun X, Yang K, Liao D, Tian L, Jiang H, Lu W. Wang J, et al. PLoS One. 2013 Jul 3;8(7):e67942. doi: 10.1371/journal.pone.0067942. Print 2013. PLoS One. 2013. PMID: 23844134 Free PMC article. - Effects of chronic exposure to cigarette smoke on canonical transient receptor potential expression in rat pulmonary arterial smooth muscle.
Wang J, Chen Y, Lin C, Jia J, Tian L, Yang K, Zhao L, Lai N, Jiang Q, Sun Y, Zhong N, Ran P, Lu W. Wang J, et al. Am J Physiol Cell Physiol. 2014 Feb 15;306(4):C364-73. doi: 10.1152/ajpcell.00048.2013. Epub 2013 Dec 11. Am J Physiol Cell Physiol. 2014. PMID: 24336649 Free PMC article. - Exploring the pathogenesis of pulmonary vascular disease.
Ejikeme C, Safdar Z. Ejikeme C, et al. Front Med (Lausanne). 2024 Jul 10;11:1402639. doi: 10.3389/fmed.2024.1402639. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39050536 Free PMC article. Review. - TRPC and TRPV Channels' Role in Vascular Remodeling and Disease.
Martín-Bórnez M, Galeano-Otero I, Del Toro R, Smani T. Martín-Bórnez M, et al. Int J Mol Sci. 2020 Aug 25;21(17):6125. doi: 10.3390/ijms21176125. Int J Mol Sci. 2020. PMID: 32854408 Free PMC article. Review.
References
- Abbott D, Comby P, Charuel C, Graepel P, Hanton G, Leblanc B, Lodola A, Longeart L, Paulus G, Peters C, Stadler J. Preclinical safety profile of sildenafil. Int J Impot Res 16: 498–504, 2004 - PubMed
- Andersen CU, Mulvany MJ, Simonsen U. Lack of synergistic effect of molsidomine and sildenafil on development of pulmonary hypertension in chronic hypoxic rats. Eur J Pharmacol 510: 87–96, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous