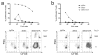Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis - PubMed (original) (raw)
Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis
Vincent Flacher et al. J Invest Dermatol. 2010 Mar.
Abstract
Antigen-presenting cells can capture antigens that are deposited in the skin, including vaccines given subcutaneously. These include different dendritic cells (DCs) such as epidermal Langerhans cells (LCs), dermal DCs, and dermal langerin+ DCs. To evaluate access of dermal antigens to skin DCs, we used mAb to two C-type lectin endocytic receptors, DEC-205/CD205 and langerin/CD207. When applied to murine and human skin explant cultures, these mAbs were efficiently taken up by epidermal LCs. In addition, anti-DEC-205 targeted langerin+ CD103+ and langerin- CD103- mouse dermal DCs. Unexpectedly, intradermal injection of either mAb, but not isotype control, resulted in strong and rapid labeling of LCs in situ, implying that large molecules can diffuse through the basement membrane into the epidermis. Epidermal LCs targeted in vivo by ovalbumin-coupled anti-DEC-205 potently presented antigen to CD4+ and CD8+ T cells in vitro. However, to our surprise, LCs targeted through langerin were unable to trigger T-cell proliferation. Thus, epidermal LCs have a major function in uptake of lectin-binding antibodies under standard vaccination conditions.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures
Figure 1. Langerhans cells in situ are specifically targeted by mAb in murine skin explant cultures
(a): Whole skin explants from BALB/c mice were incubated for 30′ to 48h in culture medium containing NLDC145 (anti-mouse DEC-205) hybridoma supernatant. Epidermis was then separated from dermis, fixed, and targeting mAb was revealed with anti-rat IgG secondary Ab. Arrowheads indicate dermal cells targeted in situ. Arrows point at DC-filled dermal lymphatic vessels. (b): Whole skin explants from BALB/c mice were incubated for 18 h in culture medium containing NLDC145 or LODNP16 (isotype control) hybridoma supernatants, then epidermis was separated, fixed, and stained with anti-rat IgG Ab. (c): Whole skin explants from BALB/c or C57BL/6 mice were incubated for 4h with 5μg/mL of the indicated purified mAb, then epidermis was separated, fixed, and stained with anti-rat IgG Ab. Scale bars = 100μm. Results are representative of three independent experiments.
Figure 2. Langerhans cells and dermal DC migrating out of murine skin explants transport the targeting mAb
Whole skin explants were incubated for 3h in medium containing L31 (anti-langerin), NLDC145 (anti-DEC-205), or IgG2a isotype control, washed in PBS and cultured for 3 days. Targeting mAb was detected in permeabilised migratory cells by anti-rat IgG Ab. Cells were counterstained with 929F3 mAb to an epitope on the cytosolic domain of langerin in (a) or 929F3 anti-langerin plus anti-CD103 in (b). Histograms in (b) show expression of the targeting mAb on subsets gated as depicted in the density plot on the left. Filled histograms: IgG2a; empty histograms: anti-DEC-205. Results are representative of three independent experiments.
Figure 3. Langerhans cells are targeted within the epidermis following intradermal injection of anti-lectin antibodies in vivo
Different targeting mAb were injected intradermally into the ear skin of C57BL/6 mice. Epidermal sheets were prepared 18 h after injection of 0.2, 1, or 5 μg of NLDC145 (a) and 24h or 5 days after injection of 1 μg IgG2a, L31, or NLDC145 (b). The capture of the injected mAb was visualized with anti-rat IgG Ab. (c): 18h after injection of 1μg targeting mAb, MHC class II (upper panel) and targeting mAb (lower panel) were revealed on epidermal sheets from langerin−/− mice. (d): 4 h after injection of 1μg targeting mAb into ear skin of BALB/c mice, whole skin explants were cultured for 3 days. The targeting mAb was detected in permeabilised migratory cells (gated on langerin+ cells; mAb 929F3). Filled histograms: IgG2a; empty histograms: mAb as indicated. Scale bars = 100μm. Results are representative of three independent experiments.
Figure 4. Langerhans cells present DEC-205–targeted antigen following in vivo uptake
4h after i.d. injection of 0.5μg of OVA-coupled anti-DEC-205, or indicated control reagents, epidermal sheets were prepared and cultured for 3 days. Migratory epidermal LC were then cultured with CFSE-labelled CD8+ (a) or CD4+ (b) T cells purified from OT-II or OT-I mice, respectively. After 4 days, T cell proliferation was evaluated by dilution of CFSE. Upper panels show T cell proliferation observed at different T/LC ratios, and lower panels display typical FACS stainings. Results are representative of two independent experiments.
Figure 5. Human skin DC bind and transport targeting mAb
Human whole skin explants were incubated for 4h in medium containing DCGM4 (anti-langerin), MG38 (anti-DEC-205), or IgG1 isotype control, then cultured for 4 days. Targeting mAb were detected in permeabilised migratory cells by means of anti-mouse IgG secondary Ab. Cells were counterstained with anti-CD1a or anti-langerin. n.d., not determined. Results are representative of two independent experiments.
Similar articles
- Skin langerin+ dendritic cells transport intradermally injected anti-DEC-205 antibodies but are not essential for subsequent cytotoxic CD8+ T cell responses.
Flacher V, Tripp CH, Haid B, Kissenpfennig A, Malissen B, Stoitzner P, Idoyaga J, Romani N. Flacher V, et al. J Immunol. 2012 Mar 1;188(5):2146-55. doi: 10.4049/jimmunol.1004120. Epub 2012 Jan 30. J Immunol. 2012. PMID: 22291181 Free PMC article. - Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance.
Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, Romani N. Flacher V, et al. EMBO Mol Med. 2014 Sep;6(9):1191-204. doi: 10.15252/emmm.201303283. EMBO Mol Med. 2014. PMID: 25085878 Free PMC article. - Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207.
Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, Kapp M, Kämmerer U, Douillard P, Kämpgen E, Koch F, Saeland S, Romani N. Stoitzner P, et al. J Invest Dermatol. 2003 Feb;120(2):266-74. doi: 10.1046/j.1523-1747.2003.12042.x. J Invest Dermatol. 2003. PMID: 12542532 - Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin.
Romani N, Clausen BE, Stoitzner P. Romani N, et al. Immunol Rev. 2010 Mar;234(1):120-41. doi: 10.1111/j.0105-2896.2009.00886.x. Immunol Rev. 2010. PMID: 20193016 Free PMC article. Review. - Langerhans cells - dendritic cells of the epidermis.
Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Romani N, et al. APMIS. 2003 Jul-Aug;111(7-8):725-40. doi: 10.1034/j.1600-0463.2003.11107805.x. APMIS. 2003. PMID: 12974775 Review.
Cited by
- Targeting skin dendritic cells to improve intradermal vaccination.
Romani N, Flacher V, Tripp CH, Sparber F, Ebner S, Stoitzner P. Romani N, et al. Curr Top Microbiol Immunol. 2012;351:113-38. doi: 10.1007/82_2010_118. Curr Top Microbiol Immunol. 2012. PMID: 21253784 Free PMC article. Review. - Macrophage- and neutrophil-derived TNF-α instructs skin langerhans cells to prime antiviral immune responses.
Epaulard O, Adam L, Poux C, Zurawski G, Salabert N, Rosenbaum P, Dereuddre-Bosquet N, Zurawski S, Flamar AL, Oh S, Romain G, Chapon C, Banchereau J, Lévy Y, Le Grand R, Martinon F. Epaulard O, et al. J Immunol. 2014 Sep 1;193(5):2416-26. doi: 10.4049/jimmunol.1303339. Epub 2014 Jul 23. J Immunol. 2014. PMID: 25057007 Free PMC article. - In vivo targeting of protein antigens to dendritic cells using anti-DEC-205 single chain antibody improves HIV Gag specific CD4+ T cell responses protecting from airway challenge with recombinant vaccinia-gag virus.
Ngu LN, Nji NN, Ambada GE, Sagnia B, Sake CN, Tchadji JC, Njambe Priso GD, Lissom A, Tchouangueu TF, Manga Tebit D, Waffo AB, Park CG, Steinman RM, Überla K, Nchinda GW. Ngu LN, et al. Immun Inflamm Dis. 2019 Jun;7(2):55-67. doi: 10.1002/iid3.151. Epub 2017 Mar 13. Immun Inflamm Dis. 2019. PMID: 28474788 Free PMC article. - Antigen targeting to dendritic cells: Still a place in future immunotherapy?
Stoitzner P, Romani N, Rademacher C, Probst HC, Mahnke K. Stoitzner P, et al. Eur J Immunol. 2022 Dec;52(12):1909-1924. doi: 10.1002/eji.202149515. Epub 2022 Jun 2. Eur J Immunol. 2022. PMID: 35598160 Free PMC article. Review. - Antigen presentation by Langerhans cells.
Igyártó BZ, Kaplan DH. Igyártó BZ, et al. Curr Opin Immunol. 2013 Feb;25(1):115-9. doi: 10.1016/j.coi.2012.11.007. Epub 2012 Dec 12. Curr Opin Immunol. 2013. PMID: 23246038 Free PMC article. Review.
References
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity. 2006;25:153–162. - PubMed
- Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. - PubMed
- Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI042848-059004/AI/NIAID NIH HHS/United States
- R01 AI040874/AI/NIAID NIH HHS/United States
- L 120/FWF_/Austrian Science Fund FWF/Austria
- AI 40874/AI/NIAID NIH HHS/United States
- AI 057158/AI/NIAID NIH HHS/United States
- AI 13013/AI/NIAID NIH HHS/United States
- R01 AI013013/AI/NIAID NIH HHS/United States
- R01 AI040874-12/AI/NIAID NIH HHS/United States
- P01 AI051573-080005/AI/NIAID NIH HHS/United States
- P01 AI051573/AI/NIAID NIH HHS/United States
- R01 AI013013-34/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials